Nuclear overhauser enhancement mediated chemical exchange saturation transfer imaging at 7 Tesla in glioblastoma patients
- PMID: 25111650
- PMCID: PMC4128651
- DOI: 10.1371/journal.pone.0104181
Nuclear overhauser enhancement mediated chemical exchange saturation transfer imaging at 7 Tesla in glioblastoma patients
Abstract
Background and purpose: Nuclear Overhauser Enhancement (NOE) mediated chemical exchange saturation transfer (CEST) is a novel magnetic resonance imaging (MRI) technique on the basis of saturation transfer between exchanging protons of tissue proteins and bulk water. The purpose of this study was to evaluate and compare the information provided by three dimensional NOE mediated CEST at 7 Tesla (7T) and standard MRI in glioblastoma patients.
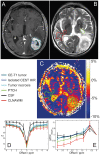
Patients and methods: Twelve patients with newly diagnosed histologically proven glioblastoma were enrolled in this prospective ethics committee-approved study. NOE mediated CEST contrast was acquired with a modified three-dimensional gradient-echo sequence and asymmetry analysis was conducted at 3.3 ppm (B1 = 0.7 µT) to calculate the magnetization transfer ratio asymmetry (MTR(asym)). Contrast enhanced T1 (CE-T1) and T2-weighted images were acquired at 3T and used for data co-registration and comparison.
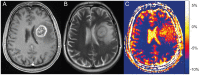
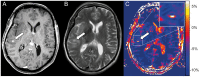
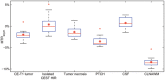
Results: Mean NOE mediated CEST signal based on MTR(asym) values over all patients was significantly increased (p<0.001) in CE-T1 tumor (-1.99 ± 1.22%), tumor necrosis (-1.36 ± 1.30%) and peritumoral CEST hyperintensities (PTCH) within T2 edema margins (-3.56 ± 1.24%) compared to contralateral normal appearing white matter (-8.38 ± 1.19%). In CE-T1 tumor (p = 0.015) and tumor necrosis (p<0.001) mean MTR(asym) values were significantly higher than in PTCH. Extent of the surrounding tumor hyperintensity was smaller in eight out of 12 patients on CEST than on T2-weighted images, while four displayed at equal size. In all patients, isolated high intensity regions (0.40 ± 2.21%) displayed on CEST within the CE-T1 tumor that were not discernible on CE-T1 or T2-weighted images.
Conclusion: NOE mediated CEST Imaging at 7 T provides additional information on the structure of peritumoral hyperintensities in glioblastoma and displays isolated high intensity regions within the CE-T1 tumor that cannot be acquired on CE-T1 or T2-weighted images. Further research is needed to determine the origin of NOE mediated CEST and possible clinical applications such as therapy assessment or biopsy planning.
Conflict of interest statement
Figures
References
-
- DeAngelis LM (2001) Brain Tumors. New England Journal of Medicine 344: 114–123. - PubMed
-
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, et al. (2010) Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. Journal of Clinical Oncology 28: 1963–1972. - PubMed
-
- Scott JN, Brasher PMA, Sevick RJ, Rewcastle NB, Forsyth PA (2002) How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 59: 947–949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

