A computational approach to evaluate the androgenic affinity of iprodione, procymidone, vinclozolin and their metabolites
- PMID: 25111804
- PMCID: PMC4128724
- DOI: 10.1371/journal.pone.0104822
A computational approach to evaluate the androgenic affinity of iprodione, procymidone, vinclozolin and their metabolites
Abstract
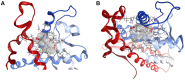
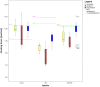
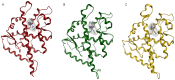
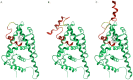
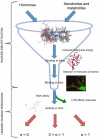
Our research is aimed at devising and assessing a computational approach to evaluate the affinity of endocrine active substances (EASs) and their metabolites towards the ligand binding domain (LBD) of the androgen receptor (AR) in three distantly related species: human, rat, and zebrafish. We computed the affinity for all the selected molecules following a computational approach based on molecular modelling and docking. Three different classes of molecules with well-known endocrine activity (iprodione, procymidone, vinclozolin, and a selection of their metabolites) were evaluated. Our approach was demonstrated useful as the first step of chemical safety evaluation since ligand-target interaction is a necessary condition for exerting any biological effect. Moreover, a different sensitivity concerning AR LBD was computed for the tested species (rat being the least sensitive of the three). This evidence suggests that, in order not to over-/under-estimate the risks connected with the use of a chemical entity, further in vitro and/or in vivo tests should be carried out only after an accurate evaluation of the most suitable cellular system or animal species. The introduction of in silico approaches to evaluate hazard can accelerate discovery and innovation with a lower economic effort than with a fully wet strategy.
Conflict of interest statement
Figures

Similar articles
-
Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p'-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat.Toxicol Ind Health. 1999 Jan-Mar;15(1-2):94-118. doi: 10.1177/074823379901500109. Toxicol Ind Health. 1999. PMID: 10188194
-
Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish.Chemosphere. 2021 Jun;272:129577. doi: 10.1016/j.chemosphere.2021.129577. Epub 2021 Jan 7. Chemosphere. 2021. PMID: 33465616
-
Cumulative and antagonistic effects of a mixture of the antiandrogens vinclozolin and iprodione in the pubertal male rat.Toxicol Sci. 2009 Sep;111(1):179-88. doi: 10.1093/toxsci/kfp137. Epub 2009 Jun 29. Toxicol Sci. 2009. PMID: 19564212
-
Partial androgen insensitivity and correlations with the predicted three dimensional structure of the androgen receptor ligand-binding domain.Mol Cell Endocrinol. 1998 Feb;137(1):41-50. doi: 10.1016/s0303-7207(97)00229-3. Mol Cell Endocrinol. 1998. PMID: 9607727 Review.
-
Cumulative effects of in utero administration of mixtures of "antiandrogens" on male rat reproductive development.Toxicol Pathol. 2009 Jan;37(1):100-13. doi: 10.1177/0192623308329478. Epub 2009 Jan 15. Toxicol Pathol. 2009. PMID: 19147833 Review.
Cited by
-
Lipase-Catalyzed Synthesis, Antioxidant Activity, Antimicrobial Properties and Molecular Docking Studies of Butyl Dihydrocaffeate.Molecules. 2022 Aug 7;27(15):5024. doi: 10.3390/molecules27155024. Molecules. 2022. PMID: 35956977 Free PMC article.
-
LRRC8A is essential for hypotonicity-, but not for DAMP-induced NLRP3 inflammasome activation.Elife. 2020 Nov 20;9:e59704. doi: 10.7554/eLife.59704. Elife. 2020. PMID: 33216713 Free PMC article.
-
The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system.Nat Commun. 2022 Feb 16;13(1):904. doi: 10.1038/s41467-022-28520-4. Nat Commun. 2022. PMID: 35173167 Free PMC article.
-
Surface Plasmon Resonance as a Tool for Ligand Binding Investigation of Engineered GPR17 Receptor, a G Protein Coupled Receptor Involved in Myelination.Front Chem. 2020 Jan 10;7:910. doi: 10.3389/fchem.2019.00910. eCollection 2019. Front Chem. 2020. PMID: 31998697 Free PMC article.
-
Chiral Recognition of Dansyl Derivatives with an Amino Acid-Based Molecular Micelle: A Molecular Dynamics Investigation.Open J Phys Chem. 2021 May;11(2):64-86. doi: 10.4236/ojpc.2021.112004. Epub 2021 May 26. Open J Phys Chem. 2021. PMID: 34123572 Free PMC article.
References
-
- European Parliament (2006) Regulation (EC) No. 1907/2006 of the European Parliament and the Council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). OJL 396.
-
- European Parliament (2009) Regulation (EC) No. 1107/2009 of the European Parliament and the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJL 309.
-
- European Parliament (1998) Directive, 1998. 98/8/EC of the European Parliament and the Council of 16 February 1998 concerning the placing on the pacing of biocidal products on the market. OJL 123.
-
- European Parliament (2009) Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products OJL. 342: 59.
-
- European Parliament (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. OJL 327: 72.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

