Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review
- PMID: 25111859
- PMCID: PMC4298376
- DOI: 10.1002/cam4.299
Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review
Abstract
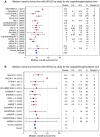
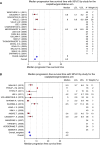
Cisplatin/gemcitabine association has been a standard of care for first-line regimen in advanced biliary tract cancer nevertheless oxaliplatin/gemcitabine regimen is frequently preferred. Because comparative effectiveness in clinical outcomes of cisplatin- versus oxaliplatin-containing chemotherapy is not available, a systematic review of studies assessing cisplatin/gemcitabine or oxaliplatin/gemcitabine chemotherapies in advanced biliary tract cancer was performed. Published studies evaluating cisplatin/gemcitabine or oxaliplatin/gemcitabine in advanced biliary tract cancer were included. Each study was weighted according to the number of patients included. The primary objective was to assess weighted median of medians overall survival (mOS) reported for both regimens. Secondary goals were to assess weighted median of medians progression-free survival (mPFS) and toxic effects were pooled and compared within each arm. Thirty-three studies involving 1470 patients were analyzed. In total, 771 and 699 patients were treated by cisplatin/gemcitabine and oxaliplatin/gemcitabine, respectively. Weighted median of mOS was 9.7 months in cisplatin group and 9.5 months in oxaliplatin group. Cisplatin-based chemotherapy was significantly associated with more grade 3 and 4 asthenia, diarrhea, liver toxicity, and hematological toxicity. Sensitivity analysis including only the studies with the standard regimen of cisplatin (25-35 mg/m(2) administered on days 1 and 8) showed that the weighted median of mOS increased from 9.7 to 11.7 months but Gem/CDDP regimen remained more toxic than Gemox regimen. These results suggest that the Gem/CDDP regimen with cisplatin (25-35 mg/m(2)) administered on days 1 and 8 is associated with survival advantage than Gemox regimen but with addition of toxicity.
Keywords: Biliary tract cancer; cisplatin; gemcitabine; oxaliplatin.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Alvaro D, Bragazzi MC, Benedetti A, Fabris L, Fava G, Invernizzi P, et al. Cholangiocarcinoma in Italy: a national survey on clinical characteristics, diagnostic modalities and treatment. Results from the “Cholangiocarcinoma” Committee of the Italian Association for the Study of Liver disease. Dis. Liver Dis. 2011;43:60–65. - PubMed
-
- Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996;7:593–600. - PubMed
-
- Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann. Oncol. 2004;15:478–483. - PubMed
-
- Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur. J. Cancer. 2005;41:398–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials