The disulfide bonding system suppresses CsgD-independent cellulose production in Escherichia coli
- PMID: 25112475
- PMCID: PMC4248800
- DOI: 10.1128/JB.02019-14
The disulfide bonding system suppresses CsgD-independent cellulose production in Escherichia coli
Abstract
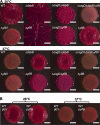
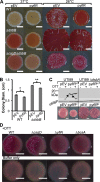
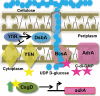
The bacterial extracellular matrix encases cells and protects them from host-related and environmental insults. The Escherichia coli master biofilm regulator CsgD is required for the production of the matrix components curli and cellulose. CsgD activates the diguanylate cyclase AdrA, which in turn stimulates cellulose production through cyclic di-GMP (c-di-GMP). Here, we identified and characterized a CsgD- and AdrA-independent cellulose production pathway that was maximally active when cultures were grown under reducing conditions or when the disulfide bonding system (DSB) was compromised. The CsgD-independent cellulose activation pathway was dependent on a second diguanylate cyclase, called YfiN. c-di-GMP production by YfiN was repressed by the periplasmic protein YfiR, and deletion of yfiR promoted CsgD-independent cellulose production. Conversely, when YfiR was overexpressed, cellulose production was decreased. Finally, we found that YfiR was oxidized by DsbA and that intraprotein YfiR disulfide bonds stabilized YfiR in the periplasm. Altogether, we showed that reducing conditions and mutations in the DSB system caused hyperactivation of YfiN and subsequent CsgD-independent cellulose production.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Dittmer CG. 1931. Animal aggregations: a study in general sociology. J. Educ. Sociol. 5:130. 10.2307/2961735. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

