Bacteriophage as effective decolonising agent for elimination of MRSA from anterior nares of BALB/c mice
- PMID: 25112504
- PMCID: PMC4236609
- DOI: 10.1186/s12866-014-0212-8
Bacteriophage as effective decolonising agent for elimination of MRSA from anterior nares of BALB/c mice
Abstract
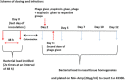
Background: Nasal carriers not only pose serious threat to themselves but also to the community by playing an active role in the dissemination of serious and life threatening S. aureus especially MRSA strains. The present study focuses on the use of broad spectrum lytic phage as decolonising agent. In addition, the combined use of lytic phage with mupirocin has also been investigated as an effective decolonising regimen. The effect of phage on the adherence, invasion and cytotoxic effect of MRSA strains on nasal epithelial cells was studied in an ex-vivo model of cultured murine nasal epithelial cells. This was followed by demonstration of therapeutic potential of phage along with mupirocin in decolonising the nares of BALB/c mice using a nasal model of MRSA colonisation.
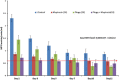
Results: Phage was able to significantly reduce the in vitro adherence, invasion and cytotoxicity of MRSA 43300 as well as other clinical MRSA strains on murine nasal epithelial cells as compared to untreated control. Also, the frequency of emergence of spontaneous mutants decreased to negligible levels when both the agents (phage and mupirocin) were used together.
Conclusion: Phage MR-10, given along with mupirocin showed an additive effect and the combination was able to effectively eradicate the colonising MRSA population from the nares of mice by day 5.
Figures



References
-
- Boyce JM, Landry M, Deetz TR, DuPont HL: Epidemiologic studies of an outbreak of nosocomial methicillin-resistantS. aureusinfections.Infect Control 1981, 2:110–116. - PubMed
-
- von Eiff C, Becker K, Machka K, Stammer H, Peters G: Hospital and community-acquired methicillin-resistantStaphylococcus aureusin Germany.Clin Microbiol Infect 2006, 12(Suppl 4):461.
-
- Weems JJ, Beck LB: Nasal carriage ofStaphylococcus aureusas a risk factor for skin and soft tissue infections.Current Infectious Disease Reports 2002, 4(5):420–425. - PubMed
-
- Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ: Eradication of methicillin-resistantStaphylococcus aureuscarriage: a systematic review.Clin Infect Dis 2009, 48:922–930. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

