Protective genotypes in HIV infection reflect superior function of KIR3DS1+ over KIR3DL1+ CD8+ T cells
- PMID: 25112829
- PMCID: PMC4500641
- DOI: 10.1038/icb.2014.68
Protective genotypes in HIV infection reflect superior function of KIR3DS1+ over KIR3DL1+ CD8+ T cells
Abstract
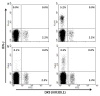
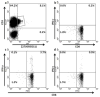
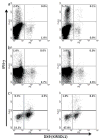
Certain human class I histocompatibility-linked leukocyte antigen (HLA)/killer cell immunoglobulin-like receptor (KIR) genotypic combinations confer more favourable prognoses upon exposure to human immunodeficiency virus (HIV). These combinations influence natural killer (NK) cell function, thereby implicating NK cells in protection from HIV infection or disease progression. Because CD8(+) T cells restrict HIV replication, depend upon HLA class I antigen presentation and can also express KIR molecules, we investigated how these HLA/KIR combinations relate to the phenotype and function of CD8(+) T cells from uninfected controls and individuals with chronic HIV infection. CD8(+) T cells from KIR3DL1 and KIR3DS1 homozygous individuals, and expressing the corresponding KIR, were enumerated and phenotyped for CD127, CD57 and CD45RA expression. Ex vivo and in vitro responsiveness to antigen-specific and polyclonal stimulation was compared between KIR-expressing and non-expressing CD8(+) T cells by interferon-γ production. There were higher numbers and fractions of KIR3DL1-expressing CD8(+) T cells in HIV-infected individuals independent of HLA-Bw4 co-expression, whereas expansion of KIR3DS1-expressing CD8(+) T cells reflected HLA-Bw4*80I co-expression. KIR3DL1(+) and S1(+) CD8(+) T cells were predominantly CD127(-)CD57(+)CD45RA(+). KIR3DL1-expressing CD8(+) T cells were insensitive to ex vivo stimulation with peptides from HIV or common viruses, but responded to anti-CD3 and recovered responsiveness to common viruses in vitro. Ex vivo non-responsiveness of KIR3DL1-expressing CD8(+) T cells was also independent of HLA-Bw4. KIR3DS1-expressing T cells responded normally to ex vivo antigenic stimulation, illustrating functional superiority over KIR3DL1(+) CD8(+) T cells.
Figures




Similar articles
-
KIR3DL1-Negative CD8 T Cells and KIR3DL1-Negative Natural Killer Cells Contribute to the Advantageous Control of Early Human Immunodeficiency Virus Type 1 Infection in HLA-B Bw4 Homozygous Individuals.Front Immunol. 2018 Aug 10;9:1855. doi: 10.3389/fimmu.2018.01855. eCollection 2018. Front Immunol. 2018. PMID: 30147699 Free PMC article.
-
Time to seroconversion in HIV-exposed subjects carrying protective versus non protective KIR3DS1/L1 and HLA-B genotypes.PLoS One. 2014 Oct 17;9(10):e110480. doi: 10.1371/journal.pone.0110480. eCollection 2014. PLoS One. 2014. PMID: 25330014 Free PMC article.
-
HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection.J Virol. 2009 Jul;83(13):6798-805. doi: 10.1128/JVI.00256-09. Epub 2009 Apr 22. J Virol. 2009. PMID: 19386717 Free PMC article.
-
Natural killer (NK) cell receptor-HLA ligand genotype combinations associated with protection from HIV infection: investigation of how protective genotypes influence anti HIV NK cell functions.AIDS Res Ther. 2017 Sep 12;14(1):38. doi: 10.1186/s12981-017-0172-9. AIDS Res Ther. 2017. PMID: 28893287 Free PMC article. Review.
-
KIR-HLA intercourse in HIV disease.Trends Microbiol. 2008 Dec;16(12):620-7. doi: 10.1016/j.tim.2008.09.002. Epub 2008 Oct 29. Trends Microbiol. 2008. PMID: 18976921 Free PMC article. Review.
Cited by
-
Natural Killer Cell Interactions with Classical and Non-Classical Human Leukocyte Antigen Class I in HIV-1 Infection.Front Immunol. 2017 Nov 14;8:1496. doi: 10.3389/fimmu.2017.01496. eCollection 2017. Front Immunol. 2017. PMID: 29184550 Free PMC article. Review.
-
KIR3DL1-Negative CD8 T Cells and KIR3DL1-Negative Natural Killer Cells Contribute to the Advantageous Control of Early Human Immunodeficiency Virus Type 1 Infection in HLA-B Bw4 Homozygous Individuals.Front Immunol. 2018 Aug 10;9:1855. doi: 10.3389/fimmu.2018.01855. eCollection 2018. Front Immunol. 2018. PMID: 30147699 Free PMC article.
-
Improved full-length killer cell immunoglobulin-like receptor transcript discovery in Mauritian cynomolgus macaques.Immunogenetics. 2017 May;69(5):325-339. doi: 10.1007/s00251-017-0977-7. Epub 2017 Mar 25. Immunogenetics. 2017. PMID: 28343239 Free PMC article.
-
Effects of the killer immunoglobulin-like receptor (KIR) polymorphisms on HIV acquisition: A meta-analysis.PLoS One. 2019 Dec 2;14(12):e0225151. doi: 10.1371/journal.pone.0225151. eCollection 2019. PLoS One. 2019. PMID: 31790432 Free PMC article.
-
The Emerging Roles of Human Leukocyte Antigen-F in Immune Modulation and Viral Infection.Front Immunol. 2019 May 10;10:964. doi: 10.3389/fimmu.2019.00964. eCollection 2019. Front Immunol. 2019. PMID: 31134067 Free PMC article. Review.
References
-
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. - PubMed
-
- Williams AP, Bateman AR, Khakoo SI. Hanging in the balance. KIR and their role in disease. Mol Interv. 2005;5:226–40. - PubMed
-
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

