Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment
- PMID: 25112870
- PMCID: PMC4175350
- DOI: 10.1074/jbc.M114.572172
Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment
Abstract
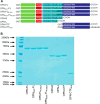
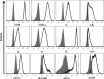
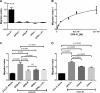
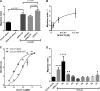
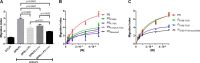
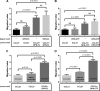
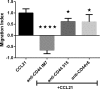
Thrombin cleavage alters the function of osteopontin (OPN) by exposing an integrin binding site and releasing a chemotactic C-terminal fragment. Here, we examined thrombin cleavage of OPN in the context of dendritic cell (DC) migration to define its functional domains. Full-length OPN (OPN-FL), thrombin-cleaved N-terminal fragment (OPN-R), thrombin- and carboxypeptidase B2-double-cleaved N-terminal fragment (OPN-L), and C-terminal fragment (OPN-CTF) did not have intrinsic chemotactic activity, but all potentiated CCL21-induced DC migration. OPN-FL possessed the highest potency, whereas OPNRAA-FL had substantially less activity, indicating the importance of RGD. We identified a conserved (168)RSKSKKFRR(176) sequence on OPN-FL that spans the thrombin cleavage site, and it demonstrated potent pro-chemotactic effects on CCL21-induced DC migration. OPN-FLR168A had reduced activity, and the double mutant OPNRAA-FLR168A had even lower activity, indicating that these functional domains accounted for most of the pro-chemotactic activity of OPN-FL. OPN-CTF also possessed substantial pro-chemotactic activity, which was fully expressed upon thrombin cleavage and its release from the intact protein, because OPN-CTF was substantially more active than OPNRAA-FLR168A containing the OPN-CTF sequence within the intact protein. OPN-R and OPN-L possessed similar potency, indicating that the newly exposed C-terminal SVVYGLR sequence in OPN-R was not involved in the pro-chemotactic effect. OPN-FL and OPN-CTF did not directly bind to the CD44 standard form or CD44v6. In conclusion, thrombin cleavage of OPN disrupts a pro-chemotactic sequence in intact OPN, and its loss of pro-chemotactic activity is compensated by the release of OPN-CTF, which assumes a new conformation and possesses substantial activity in enhancing chemokine-induced migration of DCs.
Keywords: Chemokine; Dendritic Cell; Migration; Osteopontin; Thrombin.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


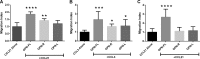
Similar articles
-
Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage.J Biol Chem. 2013 Feb 1;288(5):3097-111. doi: 10.1074/jbc.M112.362954. Epub 2012 Nov 30. J Biol Chem. 2013. PMID: 23204518 Free PMC article.
-
SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis.Mol Cell Biochem. 2015 Oct;408(1-2):191-203. doi: 10.1007/s11010-015-2495-y. Epub 2015 Jun 27. Mol Cell Biochem. 2015. PMID: 26112906
-
Osteopontin is highly susceptible to cleavage in bovine milk and the proteolytic fragments bind the αVβ₃-integrin receptor.J Dairy Sci. 2014;97(1):136-46. doi: 10.3168/jds.2013-7223. Epub 2013 Nov 21. J Dairy Sci. 2014. PMID: 24268404
-
Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases.Biomedicines. 2021 Aug 13;9(8):1006. doi: 10.3390/biomedicines9081006. Biomedicines. 2021. PMID: 34440210 Free PMC article. Review.
-
Thrombin Cleavage of Osteopontin and the Host Anti-Tumor Immune Response.Cancers (Basel). 2023 Jul 3;15(13):3480. doi: 10.3390/cancers15133480. Cancers (Basel). 2023. PMID: 37444590 Free PMC article. Review.
Cited by
-
The Multifaceted Role of Osteopontin in Prostate Pathologies.Biomedicines. 2023 Oct 26;11(11):2895. doi: 10.3390/biomedicines11112895. Biomedicines. 2023. PMID: 38001899 Free PMC article. Review.
-
Osteopontin (OPN)/SPP1: from its biochemistry to biological functions in the innate immune system and the central nervous system (CNS).Int Immunol. 2023 Apr 4;35(4):171-180. doi: 10.1093/intimm/dxac060. Int Immunol. 2023. PMID: 36525591 Free PMC article. Review.
-
Thrombin cleavage of osteopontin initiates osteopontin's tumor-promoting activity.J Thromb Haemost. 2022 May;20(5):1256-1270. doi: 10.1111/jth.15663. Epub 2022 Feb 16. J Thromb Haemost. 2022. PMID: 35108449 Free PMC article.
-
Osteopontin: A Bone-Derived Protein Involved in Rheumatoid Arthritis and Osteoarthritis Immunopathology.Biomolecules. 2023 Mar 9;13(3):502. doi: 10.3390/biom13030502. Biomolecules. 2023. PMID: 36979437 Free PMC article. Review.
-
Antibody-mediated targeting of cleavage-specific OPN-T cell interactions.PLoS One. 2019 Apr 5;14(4):e0214938. doi: 10.1371/journal.pone.0214938. eCollection 2019. PLoS One. 2019. PMID: 30951532 Free PMC article.
References
-
- Sharif S. A., Du X., Myles T., Song J. J., Price E., Lee D. M., Goodman S. B., Nagashima M., Morser J., Robinson W. H., Leung L. L. (2009) Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum. 60, 2902–2912 - PMC - PubMed
-
- Weiss J. M., Renkl A. C., Maier C. S., Kimmig M., Liaw L., Ahrens T., Kon S., Maeda M., Hotta H., Uede T., Simon J. C. (2001) Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J. Exp. Med. 194, 1219–1229 - PMC - PubMed
-
- Grassinger J., Haylock D. N., Storan M. J., Haines G. O., Williams B., Whitty G. A., Vinson A. R., Be C. L., Li S., Sørensen E. S., Tam P. P., Denhardt D. T., Sheppard D., Choong P. F., Nilsson S. K. (2009) Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with α9β1 and α4β1 integrins. Blood 114, 49–59 - PubMed
-
- Bruemmer D., Collins A. R., Noh G., Wang W., Territo M., Arias-Magallona S., Fishbein M. C., Blaschke F., Kintscher U., Graf K., Law R. E., Hsueh W. A. (2003) Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J. Clin. Invest. 112, 1318–1331 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

