Diacylglycerol kinase δ phosphorylates phosphatidylcholine-specific phospholipase C-dependent, palmitic acid-containing diacylglycerol species in response to high glucose levels
- PMID: 25112873
- PMCID: PMC4176214
- DOI: 10.1074/jbc.M114.590950
Diacylglycerol kinase δ phosphorylates phosphatidylcholine-specific phospholipase C-dependent, palmitic acid-containing diacylglycerol species in response to high glucose levels
Abstract
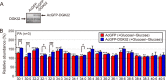
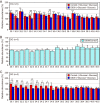
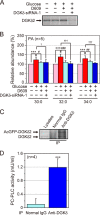
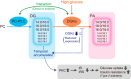
Decreased expression of diacylglycerol (DG) kinase (DGK) δ in skeletal muscles is closely related to the pathogenesis of type 2 diabetes. To identify DG species that are phosphorylated by DGKδ in response to high glucose stimulation, we investigated high glucose-dependent changes in phosphatidic acid (PA) molecular species in mouse C2C12 myoblasts using a newly established liquid chromatography/MS method. We found that the suppression of DGKδ2 expression by DGKδ-specific siRNAs significantly inhibited glucose-dependent increases in 30:0-, 32:0-, and 34:0-PA and moderately attenuated 30:1-, 32:1-, and 34:1-PA. Moreover, overexpression of DGKδ2 also enhanced the production of these PA species. MS/MS analysis revealed that these PA species commonly contain palmitic acid (16:0). D609, an inhibitor of phosphatidylcholine-specific phospholipase C (PC-PLC), significantly inhibited the glucose-stimulated production of the palmitic acid-containing PA species. Moreover, PC-PLC was co-immunoprecipitated with DGKδ2. These results strongly suggest that DGKδ preferably metabolizes palmitic acid-containing DG species supplied from the PC-PLC pathway, but not arachidonic acid (20:4)-containing DG species derived from the phosphatidylinositol turnover, in response to high glucose levels.
Keywords: Diacylglycerol; Diacylglycerol Kinase; Glucose; Palmitic Acid; Phosphatidic Acid; Phosphatidylcholine-specific Phospholipase C; Phospholipase C; Type 2 Diabetes.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Zimmet P., Alberti K. G., Shaw J. (2001) Global and societal implications of the diabetes epidemic. Nature 414, 782–787 - PubMed
-
- Biddinger S. B., Kahn C. R. (2006) From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 - PubMed
-
- Kraegen E. W., Saha A. K., Preston E., Wilks D., Hoy A. J., Cooney G. J., Ruderman N. B. (2006) Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am. J. Physiol. Endocrinol. Metab. 290, E471–E479 - PubMed
-
- Goto K., Hozumi Y., Kondo H. (2006) Diacylglycerol, phosphatidic acid, and the converting enzyme, diacylglycerol kinase, in the nucleus. Biochim. Biophys. Acta 1761, 535–541 - PubMed
-
- Mérida I., Avila-Flores A., Merino E. (2008) Diacylglycerol kinases: at the hub of cell signalling. Biochem. J. 409, 1–18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

