p53 is required for cisplatin-induced processing of the mitochondrial fusion protein L-Opa1 that is mediated by the mitochondrial metallopeptidase Oma1 in gynecologic cancers
- PMID: 25112877
- PMCID: PMC4175349
- DOI: 10.1074/jbc.M114.594812
p53 is required for cisplatin-induced processing of the mitochondrial fusion protein L-Opa1 that is mediated by the mitochondrial metallopeptidase Oma1 in gynecologic cancers
Abstract
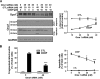
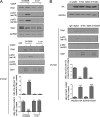
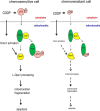
Mitochondria are highly dynamic organelles, and mitochondrial fission is a crucial step of apoptosis. Although Oma1 is believed to be responsible for long form Opa1 (L-Opa1) processing during mitochondrial fragmentation, whether and how Oma1 is involved in L-Opa1 processing and participates in the regulation of chemoresistance is unknown. Chemosensitive and chemoresistant ovarian (OVCA) and cervical (CECA) cancer cells were treated with cisplatin (CDDP). Mitochondrial dynamics and protein contents were assessed by immunofluorescence and Western blot, respectively. The requirements of Oma1 and p53 for CDDP-induced L-Opa1 processing, mitochondrial fragmentation, and apoptosis were examined by siRNA or cDNA. CDDP induces L-Opa1 processing and mitochondrial fragmentation in chemosensitive but not in chemoresistant cells. CDDP induced Oma1 40-kDa form increases in OV2008 cells, not in C13* cells. Oma1 knockdown inhibited L-Opa1 processing, mitochondrial fragmentation, and apoptosis. Silencing p53 expression attenuated the effects of CDDP in Oma1 (40 kDa) increase, L-Opa1 processing, mitochondrial fragmentation, and apoptosis in chemosensitive OVCA cells, whereas reconstitution of p53 in p53 mutant or null chemoresistant OVCA cells induced Oma1 (40 kDa) increase, L-Opa1 processing, mitochondrial fragmentation, and apoptosis irrespective of the presence of CDDP. Prohibitin 1 (Phb1) dissociates from Opa1-Phb1 complex and binds phosphorylated p53 (serine 15) in response to CDDP in chemosensitive but not chemoresistant CECA cells. These findings demonstrate that (a) p53 and Oma1 mediate L-Opa1 processing, (b) mitochondrial fragmentation is involved in CDDP-induced apoptosis in OVCA and CECA cells, and (c) dysregulated mitochondrial dynamics may in part be involved in the pathophysiology of CDDP resistance.
Keywords: Apoptosis; CDDP; Chemoresistance; Mitochondrial Fragmentation; Oma1; Opa1; Ovarian Cancer; Protein-Protein Interaction; p53.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Prohibitin 1 interacts with p53 in the regulation of mitochondrial dynamics and chemoresistance in gynecologic cancers.J Ovarian Res. 2022 Jun 7;15(1):70. doi: 10.1186/s13048-022-00999-x. J Ovarian Res. 2022. PMID: 35668443 Free PMC article.
-
Calpain-mediated processing of p53-associated parkin-like cytoplasmic protein (PARC) affects chemosensitivity of human ovarian cancer cells by promoting p53 subcellular trafficking.J Biol Chem. 2012 Feb 3;287(6):3963-75. doi: 10.1074/jbc.M111.314765. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117079 Free PMC article.
-
P73 regulates cisplatin-induced apoptosis in ovarian cancer cells via a calcium/calpain-dependent mechanism.Oncogene. 2011 Oct 13;30(41):4219-30. doi: 10.1038/onc.2011.134. Epub 2011 Apr 25. Oncogene. 2011. PMID: 21516125 Free PMC article.
-
Targeted OMA1 therapies for cancer.Int J Cancer. 2019 Nov 1;145(9):2330-2341. doi: 10.1002/ijc.32177. Epub 2019 Feb 21. Int J Cancer. 2019. PMID: 30714136 Review.
-
OPA1 processing in cell death and disease - the long and short of it.J Cell Sci. 2016 Jun 15;129(12):2297-306. doi: 10.1242/jcs.159186. Epub 2016 May 17. J Cell Sci. 2016. PMID: 27189080 Review.
Cited by
-
Altered Mitochondrial Signalling and Metabolism in Cancer.Front Oncol. 2017 Mar 20;7:43. doi: 10.3389/fonc.2017.00043. eCollection 2017. Front Oncol. 2017. PMID: 28373964 Free PMC article. Review.
-
Roles of mitochondrial fusion and fission in breast cancer progression: a systematic review.World J Surg Oncol. 2022 Oct 3;20(1):331. doi: 10.1186/s12957-022-02799-5. World J Surg Oncol. 2022. PMID: 36192752 Free PMC article.
-
The complexity of p53-mediated metabolic regulation in tumor suppression.Semin Cancer Biol. 2022 Oct;85:4-32. doi: 10.1016/j.semcancer.2021.03.010. Epub 2021 Mar 27. Semin Cancer Biol. 2022. PMID: 33785447 Free PMC article. Review.
-
Role of the mitochondrial stress response in human cancer progression.Exp Biol Med (Maywood). 2020 May;245(10):861-878. doi: 10.1177/1535370220920558. Epub 2020 Apr 23. Exp Biol Med (Maywood). 2020. PMID: 32326760 Free PMC article. Review.
-
The Role of Genetic Mutations in Mitochondrial-Driven Cancer Growth in Selected Tumors: Breast and Gynecological Malignancies.Life (Basel). 2023 Apr 12;13(4):996. doi: 10.3390/life13040996. Life (Basel). 2023. PMID: 37109525 Free PMC article. Review.
References
-
- Li J., Feng Q., Kim J. M., Schneiderman D., Liston P., Li M., Vanderhyden B., Faught W., Fung M. F., Senterman M., Korneluk R. G., Tsang B. K. (2001) Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology 142, 370–380 - PubMed
-
- Kigawa J., Sato S., Shimada M., Takahashi M., Itamochi H., Kanamori Y., Terakawa N. (2001) p53 gene status and chemosensitivity in ovarian cancer. Hum. Cell 14, 165–171 - PubMed
-
- Yang X., Fraser M., Moll U. M., Basak A., Tsang B. K. (2006) Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 66, 3126–3136 - PubMed
-
- Otera H., Ishihara N., Mihara K. (2013) New insights into the function and regulation of mitochondrial fission. Biochim. Biophys. Acta 1833, 1256–1268 - PubMed
-
- Shutt T. E., McBride H. M. (2013) Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim. Biophys. Acta 1833, 417–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

