Plakophilin-2 promotes tumor development by enhancing ligand-dependent and -independent epidermal growth factor receptor dimerization and activation
- PMID: 25113560
- PMCID: PMC4187709
- DOI: 10.1128/MCB.00758-14
Plakophilin-2 promotes tumor development by enhancing ligand-dependent and -independent epidermal growth factor receptor dimerization and activation
Abstract
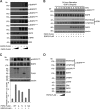
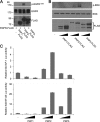
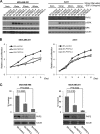
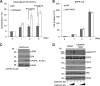
Epidermal growth factor (EGF) receptor (EGFR) has been implicated in tumor development and invasion. Dimerization and autophosphorylation of EGFR are the critical events for EGFR activation. However, the regulation of EGF-dependent and EGF-independent dimerization and phosphorylation of EGFR has not been fully understood. Here, we report that cytoplasmic protein plakophilin-2 (PKP2) is a novel positive regulator of EGFR signaling. PKP2 specifically interacts with EGFR via its N-terminal head domain. Increased PKP2 expression enhances EGF-dependent and EGF-independent EGFR dimerization and phosphorylation. Moreover, PKP2 knockdown reduces EGFR phosphorylation and attenuates EGFR-mediated signal activation, resulting in a significant decrease in proliferation and migration of cancer cells and tumor development. Our results indicate that PKP2 is a novel activator of the EGFR signaling pathway and a potential new drug target for inhibiting tumor growth.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Comment in
-
Plakophilin-2 promotes activation of epidermal growth factor receptor.Mol Cell Biol. 2014 Oct;34(20):3778-9. doi: 10.1128/MCB.00968-14. Epub 2014 Aug 11. Mol Cell Biol. 2014. PMID: 25113561 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
