The social amoeba Polysphondylium pallidum loses encystation and sporulation, but can still erect fruiting bodies in the absence of cellulose
- PMID: 25113829
- PMCID: PMC4210663
- DOI: 10.1016/j.protis.2014.07.003
The social amoeba Polysphondylium pallidum loses encystation and sporulation, but can still erect fruiting bodies in the absence of cellulose
Abstract
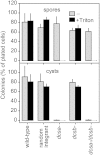
Amoebas and other freely moving protists differentiate into walled cysts when exposed to stress. As cysts, amoeba pathogens are resistant to biocides, preventing treatment and eradication. Lack of gene modification procedures has left the mechanisms of encystation largely unexplored. Genetically tractable Dictyostelium discoideum amoebas require cellulose synthase for formation of multicellular fructifications with cellulose-rich stalk and spore cells. Amoebas of its distant relative Polysphondylium pallidum (Ppal), can additionally encyst individually in response to stress. Ppal has two cellulose synthase genes, DcsA and DcsB, which we deleted individually and in combination. Dcsa- mutants formed fruiting bodies with normal stalks, but their spore and cyst walls lacked cellulose, which obliterated stress-resistance of spores and rendered cysts entirely non-viable. A dcsa-/dcsb- mutant made no walled spores, stalk cells or cysts, although simple fruiting structures were formed with a droplet of amoeboid cells resting on an sheathed column of decaying cells. DcsB is expressed in prestalk and stalk cells, while DcsA is additionally expressed in spores and cysts. We conclude that cellulose is essential for encystation and that cellulose synthase may be a suitable target for drugs to prevent encystation and render amoeba pathogens susceptible to conventional antibiotics.
Keywords: Acanthamoeba keratitis; Amoebozoa; Encystation; Polysphondylium pallidum.; cell wall biosynthesis; cellulose synthase.
Copyright © 2014 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




Similar articles
-
The cyclic AMP phosphodiesterase RegA critically regulates encystation in social and pathogenic amoebas.Cell Signal. 2014 Feb;26(2):453-9. doi: 10.1016/j.cellsig.2013.10.008. Epub 2013 Oct 31. Cell Signal. 2014. PMID: 24184654 Free PMC article.
-
Activated cAMP receptors switch encystation into sporulation.Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):7089-94. doi: 10.1073/pnas.0901617106. Epub 2009 Apr 14. Proc Natl Acad Sci U S A. 2009. PMID: 19369200 Free PMC article.
-
Cross-species functional complementation of cellulose synthase during the development of cellular slime molds.Dev Growth Differ. 2014 Sep;56(7):526-33. doi: 10.1111/dgd.12153. Epub 2014 Sep 11. Dev Growth Differ. 2014. PMID: 25208883
-
Encystation: the most prevalent and underinvestigated differentiation pathway of eukaryotes.Microbiology (Reading). 2018 May;164(5):727-739. doi: 10.1099/mic.0.000653. Epub 2018 Apr 5. Microbiology (Reading). 2018. PMID: 29620506 Review.
-
Cooperation and conflict in the social amoeba Dictyostelium discoideum.Int J Dev Biol. 2019;63(8-9-10):371-382. doi: 10.1387/ijdb.190158jm. Int J Dev Biol. 2019. PMID: 31840776 Review.
Cited by
-
Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement.Pathogens. 2025 Mar 10;14(3):268. doi: 10.3390/pathogens14030268. Pathogens. 2025. PMID: 40137753 Free PMC article. Review.
-
A conserved signalling pathway for amoebozoan encystation that was co-opted for multicellular development.Sci Rep. 2015 Apr 16;5:9644. doi: 10.1038/srep09644. Sci Rep. 2015. PMID: 25881075 Free PMC article.
References
-
- Aqeel Y., Siddiqui R., Khan N.A. Silencing of xylose isomerase and cellulose synthase by siRNA inhibits encystation in Acanthamoeba castellanii. Parasitol Res. 2013;112:1221–1227. - PubMed
-
- Bush K. Antimicrobial agents targeting bacterial cell walls and cell membranes. Rev Sci Tech. 2012;31:43–56. - PubMed
-
- Clarke M., Lohan A.J., Liu B., Lagkouvardos I., Roy S., Zafar N., Bertelli C., Schilde C., Kianianmomeni A., Burglin T.R., Frech C., Turcotte B., Kopec K.O., Synnott J.M., Choo C., Paponov I., Finkler A., Heng Tan C.S., Hutchins A.P., Weinmeier T., Rattei T., Chu J.S., Gimenez G., Irimia M., Rigden D.J., Fitzpatrick D.A., Lorenzo-Morales J., Bateman A., Chiu C.H., Tang P., Hegemann P., Fromm H., Raoult D., Greub G., Miranda-Saavedra D., Chen N., Nash P., Ginger M.L., Horn M., Schaap P., Caler L., Loftus B.J. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013;14:R11. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

