HIV-1 Vpu mediated downregulation of CD155 requires alanine residues 10, 14 and 18 of the transmembrane domain
- PMID: 25113908
- PMCID: PMC4172918
- DOI: 10.1016/j.virol.2014.07.034
HIV-1 Vpu mediated downregulation of CD155 requires alanine residues 10, 14 and 18 of the transmembrane domain
Abstract
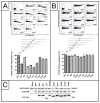
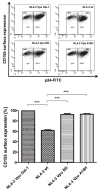
HIV-1 NL4-3 Vpu induces downregulation of cell surface CD155, a ligand for the DNAM-1 activating receptor of NK and CD8(+) T cells, to evade NK cell mediated immune response. Here we show that the conserved alanine residues at positions 10, 14 and 18 in the TM domain of Vpu are required for the efficient downregulation of cell surface CD155. In contrast, the CK-2 phosphorylation sites and the second α-helix in the cytoplasmic Vpu domain have no influence on the surface expression of CD155. Thus, compared to Vpu׳s effect on CD4, NTB-A and tetherin, the Vpu mediated downregulation of CD155 is an independent Vpu function. We finally show that in contrast to other lentiviral strains, only Vpu and Nef from HIV-1 M NL4-3 potently interfere with CD155 surface expression. Thus, Vpu seems to subvert NK cell responses against HIV-1 infected T cells by modulation of receptors necessary for NK cell activation.
Keywords: CD155; HIV-1; Nef; SIV; Vpu.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR.J Virol. 2012 Apr;86(8):4496-504. doi: 10.1128/JVI.05788-11. Epub 2012 Feb 1. J Virol. 2012. PMID: 22301152 Free PMC article.
-
Functional antagonism of rhesus macaque and chimpanzee BST-2 by HIV-1 Vpu is mediated by cytoplasmic domain interactions.J Virol. 2013 Dec;87(24):13825-36. doi: 10.1128/JVI.02567-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109238 Free PMC article.
-
CD155 on HIV-Infected Cells Is Not Modulated by HIV-1 Vpu and Nef but Synergizes with NKG2D Ligands to Trigger NK Cell Lysis of Autologous Primary HIV-Infected Cells.AIDS Res Hum Retroviruses. 2017 Feb;33(2):93-100. doi: 10.1089/AID.2015.0375. Epub 2016 Jul 20. AIDS Res Hum Retroviruses. 2017. PMID: 27296670 Free PMC article.
-
Modulation of HIV-1-host interaction: role of the Vpu accessory protein.Retrovirology. 2010 Dec 22;7:114. doi: 10.1186/1742-4690-7-114. Retrovirology. 2010. PMID: 21176220 Free PMC article. Review.
-
Transmembrane interactions of HIV-1 Vpu and tetherin.Curr HIV Res. 2012 Jun;10(4):292-7. doi: 10.2174/157016212800792450. Curr HIV Res. 2012. PMID: 22524177 Review.
Cited by
-
Novel Compound Inhibitors of HIV-1NL4-3 Vpu.Viruses. 2022 Apr 15;14(4):817. doi: 10.3390/v14040817. Viruses. 2022. PMID: 35458546 Free PMC article.
-
HIV-1 Vpu Downregulates Tim-3 from the Surface of Infected CD4+ T Cells.J Virol. 2020 Mar 17;94(7):e01999-19. doi: 10.1128/JVI.01999-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941771 Free PMC article.
-
A Natural Impact: NK Cells at the Intersection of Cancer and HIV Disease.Front Immunol. 2019 Aug 14;10:1850. doi: 10.3389/fimmu.2019.01850. eCollection 2019. Front Immunol. 2019. PMID: 31474977 Free PMC article. Review.
-
TIGIT blockade enhances NK cell activity against autologous HIV-1-infected CD4+ T cells.Clin Transl Immunology. 2021 Oct 19;10(10):e1348. doi: 10.1002/cti2.1348. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34707863 Free PMC article.
-
Vpu Protein: The Viroporin Encoded by HIV-1.Viruses. 2015 Aug 4;7(8):4352-68. doi: 10.3390/v7082824. Viruses. 2015. PMID: 26247957 Free PMC article. Review.
References
-
- Bolduan S, Votteler J, Lodermeyer V, Greiner T, Koppensteiner H, Schindler M, Thiel G, Schubert U. Ion channel activity of HIV-1 Vpu is dispensable for counteraction of CD317. Virology. 2011;416:75–85. - PubMed
-
- Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

