Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD
- PMID: 25114038
- PMCID: PMC4170611
- DOI: 10.18632/oncotarget.2137
Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD
Abstract
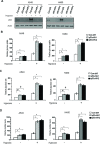
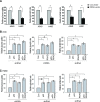
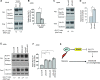
Cancer cells display enhanced glycolysis to meet their energetic and biosynthetic demands even under normal oxygen concentrations. Recent studies have revealed that tumor suppressor p53 represses glycolysis under normoxia as a novel mechanism for tumor suppression. As a common microenvironmental stress for tumors, hypoxia drives the metabolic switch from the oxidative phosphorylation to glycolysis, which is crucial for survival and proliferation of cancer cells under hypoxia. The p53's role and mechanism in regulating glycolysis under hypoxia is poorly understood. Here, we found that p53 represses hypoxia-stimulated glycolysis in cancer cells through RRAD, a newly-identified p53 target. RRAD expression is frequently decreased in lung cancer. Ectopic expression of RRAD greatly reduces glycolysis whereas knockdown of RRAD promotes glycolysis in lung cancer cells. Furthermore, RRAD represses glycolysis mainly through inhibition of GLUT1 translocation to the plasma membrane. Under hypoxic conditions, p53 induces RRAD, which in turn inhibits the translocation of GLUT1 and represses glycolysis in lung cancer cells. Blocking RRAD by siRNA greatly abolishes p53's function in repressing glycolysis under hypoxia. Taken together, our results revealed an important role and mechanism of p53 in antagonizing the stimulating effect of hypoxia on glycolysis, which contributes to p53's function in tumor suppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
RRAD inhibits the Warburg effect through negative regulation of the NF-κB signaling.Oncotarget. 2015 Jun 20;6(17):14982-92. doi: 10.18632/oncotarget.3719. Oncotarget. 2015. PMID: 25893381 Free PMC article.
-
RRAD inhibits aerobic glycolysis, invasion, and migration and is associated with poor prognosis in hepatocellular carcinoma.Tumour Biol. 2016 Apr;37(4):5097-105. doi: 10.1007/s13277-015-4329-7. Epub 2015 Nov 6. Tumour Biol. 2016. PMID: 26546438
-
Inhibition of glycolytic enzymes mediated by pharmacologically activated p53: targeting Warburg effect to fight cancer.J Biol Chem. 2011 Dec 2;286(48):41600-41615. doi: 10.1074/jbc.M111.240812. Epub 2011 Aug 23. J Biol Chem. 2011. PMID: 21862591 Free PMC article.
-
Metabolic regulation by p53: Implications for cancer therapy.Mol Cells. 2025 Apr;48(4):100198. doi: 10.1016/j.mocell.2025.100198. Epub 2025 Feb 20. Mol Cells. 2025. PMID: 39986611 Free PMC article. Review.
-
Friend or Foe: Regulation, Downstream Effectors of RRAD in Cancer.Biomolecules. 2023 Mar 5;13(3):477. doi: 10.3390/biom13030477. Biomolecules. 2023. PMID: 36979412 Free PMC article. Review.
Cited by
-
LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2.EBioMedicine. 2019 Mar;41:200-213. doi: 10.1016/j.ebiom.2019.01.035. Epub 2019 Feb 19. EBioMedicine. 2019. PMID: 30796006 Free PMC article.
-
Role of multifaceted regulators in cancer glucose metabolism and their clinical significance.Oncotarget. 2016 May 24;7(21):31572-85. doi: 10.18632/oncotarget.7765. Oncotarget. 2016. PMID: 26934324 Free PMC article. Review.
-
Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications.J Hematol Oncol. 2022 Nov 1;15(1):160. doi: 10.1186/s13045-022-01358-5. J Hematol Oncol. 2022. PMID: 36319992 Free PMC article. Review.
-
Breathing new insights into the role of mutant p53 in lung cancer.Oncogene. 2025 Feb;44(3):115-129. doi: 10.1038/s41388-024-03219-6. Epub 2024 Nov 20. Oncogene. 2025. PMID: 39567755 Free PMC article. Review.
-
Exploring the metabolic alterations in cervical cancer induced by HPV oncoproteins: From mechanisms to therapeutic targets.Biochim Biophys Acta Rev Cancer. 2025 Apr;1880(2):189292. doi: 10.1016/j.bbcan.2025.189292. Epub 2025 Mar 2. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40037419 Free PMC article. Review.
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. - PubMed
-
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. - PubMed
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137(3):413–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

