Masking of β(1-3)-glucan in the cell wall of Candida albicans from detection by innate immune cells depends on phosphatidylserine
- PMID: 25114110
- PMCID: PMC4187869
- DOI: 10.1128/IAI.01612-14
Masking of β(1-3)-glucan in the cell wall of Candida albicans from detection by innate immune cells depends on phosphatidylserine
Abstract
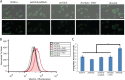
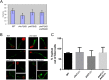
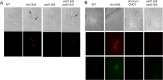
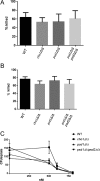
The virulence of Candida albicans in a mouse model of invasive candidiasis is dependent on the phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE). Disruption of the PS synthase gene CHO1 (i.e., cho1Δ/Δ) eliminates PS and blocks the de novo pathway for PE biosynthesis. In addition, the cho1Δ/Δ mutant's ability to cause invasive disease is severely compromised. The cho1Δ/Δ mutant also exhibits cell wall defects, and in this study, it was determined that loss of PS results in decreased masking of cell wall β(1-3)-glucan from the immune system. In wild-type C. albicans, the outer mannan layer of the wall masks the inner layer of β(1-3)-glucan from exposure and detection by innate immune effector molecules like the C-type signaling lectin Dectin-1, which is found on macrophages, neutrophils, and dendritic cells. The cho1Δ/Δ mutant exhibits increases in exposure of β(1-3)-glucan, which leads to greater binding by Dectin-1 in both yeast and hyphal forms. The unmasking of β(1-3)-glucan also results in increased elicitation of TNF-α from macrophages in a Dectin-1-dependent manner. The role of phospholipids in fungal pathogenesis is an emerging field, and this is the first study showing that loss of PS in C. albicans results in decreased masking of β(1-3)-glucan, which may contribute to our understanding of fungus-host interactions.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
β-(1,3)-Glucan Unmasking in Some Candida albicans Mutants Correlates with Increases in Cell Wall Surface Roughness and Decreases in Cell Wall Elasticity.Infect Immun. 2016 Dec 29;85(1):e00601-16. doi: 10.1128/IAI.00601-16. Print 2017 Jan. Infect Immun. 2016. PMID: 27849179 Free PMC article.
-
Exposure of Candida albicans β (1,3)-glucan is promoted by activation of the Cek1 pathway.PLoS Genet. 2019 Jan 31;15(1):e1007892. doi: 10.1371/journal.pgen.1007892. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30703081 Free PMC article.
-
Control of β-glucan exposure by the endo-1,3-glucanase Eng1 in Candida albicans modulates virulence.PLoS Pathog. 2022 Jan 7;18(1):e1010192. doi: 10.1371/journal.ppat.1010192. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 34995333 Free PMC article.
-
Face/Off: The Interchangeable Side of Candida Albicans.Front Cell Infect Microbiol. 2020 Jan 28;9:471. doi: 10.3389/fcimb.2019.00471. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32047726 Free PMC article. Review.
-
[Contribution of dectin-1 to the recognition of fungal cell wall products and the activation of innate immune response].Nihon Ishinkin Gakkai Zasshi. 2006;47(3):185-94. doi: 10.3314/jjmm.47.185. Nihon Ishinkin Gakkai Zasshi. 2006. PMID: 16940953 Review. Japanese.
Cited by
-
Assessment of the pulmonary adaptive immune response to Cladosporium cladosporioides infection using an experimental mouse model.Sci Rep. 2021 Jan 13;11(1):909. doi: 10.1038/s41598-020-79642-y. Sci Rep. 2021. PMID: 33441700 Free PMC article.
-
Cek1 regulates ß(1,3)-glucan exposure through calcineurin effectors in Candida albicans.PLoS Genet. 2022 Sep 19;18(9):e1010405. doi: 10.1371/journal.pgen.1010405. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36121853 Free PMC article.
-
Candida albicans Cannot Acquire Sufficient Ethanolamine from the Host To Support Virulence in the Absence of De Novo Phosphatidylethanolamine Synthesis.Infect Immun. 2018 Jul 23;86(8):e00815-17. doi: 10.1128/IAI.00815-17. Print 2018 Aug. Infect Immun. 2018. PMID: 29866908 Free PMC article.
-
Innovations in Antifungal Drug Discovery among Cell Envelope Synthesis Enzymes through Structural Insights.J Fungi (Basel). 2024 Feb 22;10(3):171. doi: 10.3390/jof10030171. J Fungi (Basel). 2024. PMID: 38535180 Free PMC article. Review.
-
Pathways That Synthesize Phosphatidylethanolamine Impact Candida albicans Hyphal Length and Cell Wall Composition through Transcriptional and Posttranscriptional Mechanisms.Infect Immun. 2020 Feb 20;88(3):e00480-19. doi: 10.1128/IAI.00480-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31792076 Free PMC article.
References
-
- Cassone A, Cauda R. 2012. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS 26:1457–1472 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

