Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients
- PMID: 25114118
- PMCID: PMC4249348
- DOI: 10.1128/IAI.01969-14
Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients
Abstract
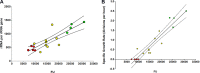
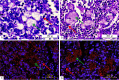
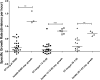
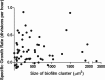
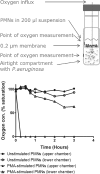
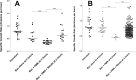
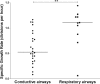
Cystic fibrosis (CF) patients have increased susceptibility to chronic lung infections by Pseudomonas aeruginosa, but the ecophysiology within the CF lung during infections is poorly understood. The aim of this study was to elucidate the in vivo growth physiology of P. aeruginosa within lungs of chronically infected CF patients. A novel, quantitative peptide nucleic acid (PNA) fluorescence in situ hybridization (PNA-FISH)-based method was used to estimate the in vivo growth rates of P. aeruginosa directly in lung tissue samples from CF patients and the growth rates of P. aeruginosa in infected lungs in a mouse model. The growth rate of P. aeruginosa within CF lungs did not correlate with the dimensions of bacterial aggregates but showed an inverse correlation to the concentration of polymorphonuclear leukocytes (PMNs) surrounding the bacteria. A growth-limiting effect on P. aeruginosa by PMNs was also observed in vitro, where this limitation was alleviated in the presence of the alternative electron acceptor nitrate. The finding that P. aeruginosa growth patterns correlate with the number of surrounding PMNs points to a bacteriostatic effect by PMNs via their strong O2 consumption, which slows the growth of P. aeruginosa in infected CF lungs. In support of this, the growth of P. aeruginosa was significantly higher in the respiratory airways than in the conducting airways of mice. These results indicate a complex host-pathogen interaction in chronic P. aeruginosa infection of the CF lung whereby PMNs slow the growth of the bacteria and render them less susceptible to antibiotic treatment while enabling them to persist by anaerobic respiration.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Alhede M, Bjarnsholt T, Jensen PO, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Hoiby N, Rasmussen TB, Givskov M. 2009. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155:3500–3508. 10.1099/mic.0.031443-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

