A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial Spiroindolone KAE609 (Cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers
- PMID: 25114127
- PMCID: PMC4187895
- DOI: 10.1128/AAC.03393-14
A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial Spiroindolone KAE609 (Cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers
Abstract
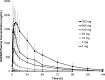
This first-in-human randomized, double-blind, placebo-controlled, ascending-single and -multiple oral dose study was designed to evaluate the safety, tolerability, and pharmacokinetics in healthy volunteers of KAE609 (cipargamin; formerly NITD609), a spiroindolone now in trials for malaria treatment. It was studied in single-dose cohorts (1 to 300 mg, including one 30-mg food effect cohort) with 4 to 10 subjects in each cohort and in multiple-dose cohorts (10 to 150 mg once daily for 3 days) with 8 subjects in each cohort. The follow-up period was 6 to 8 days post-last dose. Safety and pharmacokinetics were assessed at scheduled time points during the study. Systemic exposure in terms of the area under the concentration-time curve from 0 h extrapolated to infinity (AUC0-∞) increased in a dose-proportional manner over the dose range of 1 to 300 mg. The AUC from time zero to the time of the last quantifiable concentration (AUClast) and the maximum concentration of drug in plasma (Cmax) also increased in an approximately dose-proportional manner. When administered daily for 3 days, the accumulation ratio on day 3 (the AUC from time zero to 24 h postdosing [AUC0-24] on day 3/AUC0-24 on day 1) was in the range of 1.5 to 2 in the studied dose range (10 to 150 mg) and was consistent with an elimination half-life of around 24 h. Urine analysis for unchanged KAE609 revealed negligible amounts (≤0.01%) were excreted renally. The high fat food intake did not affect the extent of KAE609 absorption (AUC); however, the Cmax was reduced by around 27%. KAE609 was tolerated in this study, with transient gastrointestinal and genitourinary adverse events of mild to moderate intensity (semen discoloration, diarrhea, nausea and abdominal discomfort, dizziness and headache, catheter site hematoma). Gastrointestinal and genitourinary adverse events increased with rising doses.
Copyright © 2014 Leong et al.
Figures

References
-
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467. 10.1056/NEJMoa0808859 - DOI - PMC - PubMed
-
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, MCler Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. 10.1016/S0140-6736(12)60484-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

