Bioengineered functional brain-like cortical tissue
- PMID: 25114234
- PMCID: PMC4183301
- DOI: 10.1073/pnas.1324214111
Bioengineered functional brain-like cortical tissue
Abstract
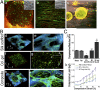
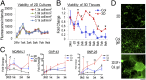
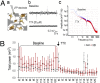
The brain remains one of the most important but least understood tissues in our body, in part because of its complexity as well as the limitations associated with in vivo studies. Although simpler tissues have yielded to the emerging tools for in vitro 3D tissue cultures, functional brain-like tissues have not. We report the construction of complex functional 3D brain-like cortical tissue, maintained for months in vitro, formed from primary cortical neurons in modular 3D compartmentalized architectures with electrophysiological function. We show that, on injury, this brain-like tissue responds in vitro with biochemical and electrophysiological outcomes that mimic observations in vivo. This modular 3D brain-like tissue is capable of real-time nondestructive assessments, offering previously unidentified directions for studies of brain homeostasis and injury.
Keywords: connectivity; electrophysiology; scaffold; silk; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Toward in vitro models of brain structure and function.Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):13682-3. doi: 10.1073/pnas.1414484111. Epub 2014 Sep 5. Proc Natl Acad Sci U S A. 2014. PMID: 25192937 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

