Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes
- PMID: 25114252
- PMCID: PMC4151774
- DOI: 10.1073/pnas.1408888111
Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes
Abstract
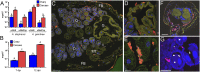
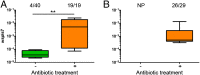
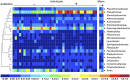
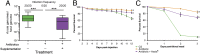
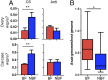
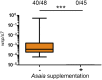
Over evolutionary time, Wolbachia has been repeatedly transferred between host species contributing to the widespread distribution of the symbiont in arthropods. For novel infections to be maintained, Wolbachia must infect the female germ line after being acquired by horizontal transfer. Although mechanistic examples of horizontal transfer exist, there is a poor understanding of factors that lead to successful vertical maintenance of the acquired infection. Using Anopheles mosquitoes (which are naturally uninfected by Wolbachia) we demonstrate that the native mosquito microbiota is a major barrier to vertical transmission of a horizontally acquired Wolbachia infection. After injection into adult Anopheles gambiae, some strains of Wolbachia invade the germ line, but are poorly transmitted to the next generation. In Anopheles stephensi, Wolbachia infection elicited massive blood meal-induced mortality, preventing development of progeny. Manipulation of the mosquito microbiota by antibiotic treatment resulted in perfect maternal transmission at significantly elevated titers of the wAlbB Wolbachia strain in A. gambiae, and alleviated blood meal-induced mortality in A. stephensi enabling production of Wolbachia-infected offspring. Microbiome analysis using high-throughput sequencing identified that the bacterium Asaia was significantly reduced by antibiotic treatment in both mosquito species. Supplementation of an antibiotic-resistant mutant of Asaia to antibiotic-treated mosquitoes completely inhibited Wolbachia transmission and partly contributed to blood meal-induced mortality. These data suggest that the components of the native mosquito microbiota can impede Wolbachia transmission in Anopheles. Incompatibility between the microbiota and Wolbachia may in part explain why some hosts are uninfected by this endosymbiont in nature.
Keywords: competitive exclusion; dysbiosis; holobiome; malaria; microbe–microbe interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

