Perifornical hypothalamic orexin and serotonin modulate the counterregulatory response to hypoglycemic and glucoprivic stimuli
- PMID: 25114294
- PMCID: PMC4274798
- DOI: 10.2337/db14-0671
Perifornical hypothalamic orexin and serotonin modulate the counterregulatory response to hypoglycemic and glucoprivic stimuli
Abstract
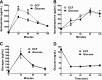
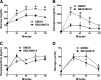
Previous reports suggested an important role for serotonin (5-hydroxytryptamine [5-HT]) in enhancing the counterregulatory response (CRR) to hypoglycemia. To elucidate the sites of action mediating this effect, we initially found that insulin-induced hypoglycemia stimulates 5-HT release in widespread forebrain regions, including the perifornical hypothalamus (PFH; 30%), ventromedial hypothalamus (34%), paraventricular hypothalamus (34%), paraventricular thalamic nucleus (64%), and cerebral cortex (63%). Of these, we focused on the PFH because of its known modulation of diverse neurohumoral and behavioral responses. In awake, behaving rats, bilateral PFH glucoprivation with 5-thioglucose stimulated adrenal medullary epinephrine (Epi) release (3,153%) and feeding (400%), while clamping PFH glucose at postprandial brain levels blunted the Epi response to hypoglycemia by 30%. The PFH contained both glucose-excited (GE) and glucose-inhibited (GI) neurons; GE neurons were primarily excited, while GI neurons were equally excited or inhibited by 5-HT at hypoglycemic glucose levels in vitro. Also, 5-HT stimulated lactate production by cultured hypothalamic astrocytes. Depleting PFH 5-HT blunted the Epi (but not feeding) response to focal PFH (69%) and systemic glucoprivation (39%), while increasing PFH 5-HT levels amplified the Epi response to hypoglycemia by 32%. Finally, the orexin 1 receptor antagonist SB334867A attenuated both the Epi (65%) and feeding (47%) responses to focal PFH glucoprivation. Thus we have identified the PFH as a glucoregulatory region where both 5-HT and orexin modulate the CRR and feeding responses to glucoprivation.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures





References
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002;45:937–948 - PubMed
-
- Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991;40:223–226 - PubMed
-
- Cryer PE. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes 1992;41:255–260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

