Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus
- PMID: 25114378
- PMCID: PMC4121042
- DOI: 10.1155/2014/701094
Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus
Abstract
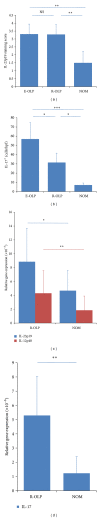
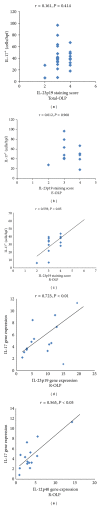
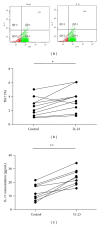
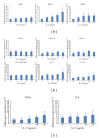
Interleukin- (IL-) 23/IL-17 axis is a newly discovered proinflammatory signaling pathway and has been implicated in the pathogenesis of many chronic inflammatory and immune disorders. Here we investigated whether the IL-23/IL-17 axis was present and functional in the lesions of oral lichen planus (OLP), a chronic inflammatory disease affecting the oral mucosa. Using immunohistochemistry and quantitative PCR, we found that the subunits of IL-23 and IL-17 were overexpressed in OLP lesions than in normal oral mucosa tissues. In addition, the expressions of IL-23 and IL-17 are positively correlated in reticular OLP tissues. Results from in vitro studies revealed that exogenous IL-23 could increase the percentage of Th17 cells and IL-17 production in the CD4+T cells from reticular OLP patients. Furthermore, we also found that exogenous IL-17 could significantly enhance the mRNA expressions of β-defensin-2, -3, CCL-20, IL-8, and TNF-α, but not β-defensin-1, CXCL-9, -10, -11, CCL-5, and IL-6 in human oral keratinocytes. Taken together, our results revealed an overexpression pattern and selectively regulatory roles of IL-23/IL-17 axis in the OLP lesions, suggesting that it may be a pivotal regulatory pathway in the complex immune network of OLP lesions.
Figures

References
-
- Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2005;100(1):40–51. - PubMed
-
- Sugerman PB, Savage NW, Walsh LJ, et al. The pathogenesis of oral lichen planus. Critical Reviews in Oral Biology and Medicine. 2002;13(4):350–365. - PubMed
-
- Eisen D, Carrozzo M, Sebastian J-VB, Thongprasom K. Number V. Oral lichen planus: clinical features and management. Oral Diseases. 2005;11(6):338–349. - PubMed
-
- Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus—a review. Journal of Oral Pathology and Medicine. 2010;39(10):729–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

