Clinical utility of erlotinib for the treatment of non-small-cell lung cancer in Japanese patients: current evidence
- PMID: 25114510
- PMCID: PMC4124069
- DOI: 10.2147/DDDT.S50358
Clinical utility of erlotinib for the treatment of non-small-cell lung cancer in Japanese patients: current evidence
Abstract
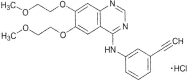
Gefitinib, an epidermal growth factor tyrosine kinase inhibitor (EGFR-TKI), has been approved in Japan for the treatment of patients with advanced non-small-cell lung cancer (NSCLC) based on Phase II clinical trials since 2002. Erlotinib, another EGFR-TKI, was also approved a few years thereafter. In 2004, activating mutations in the EGFR gene were discovered to be a predictive biomarker for EGFR-TKI treatment, and gefitinib, which is not effective for patients with EGFR wild-type NSCLC, has since been used only in patients with EGFR-mutated NSCLC. In contrast, erlotinib is potentially effective for the treatment of EGFR wild-type NSCLC. Similar to gefitinib, erlotinib is also effective for EGFR-mutated NSCLC and has been used as an initial treatment for patients with advanced EGFR-mutated NSCLC. Both gefitinib and erlotinib can be used in a Japanese clinical setting. The approved daily dose of erlotinib (150 mg) is equal to the maximum tolerated dose of erlotinib. In contrast, the daily dose of gefitinib has been set at 250 mg, which is approximately one-third of the maximum tolerated dose of gefitinib. Accordingly, a higher serum concentration can be achieved using erlotinib, compared with gefitinib. This advantage can be applied to the treatment of central nervous system metastases (brain metastasis and carcinomatous meningitis), the treatment of which is complicated by the difficulty drugs have penetrating the blood-brain barrier. Although patients with EGFR-mutated NSCLC respond dramatically to EGFR-TKIs, some patients have a poor response and the majority eventually undergo disease progression. To overcome such resistance, several novel treatment strategies, such as combination therapy and next-generation EGFR-TKIs, have been attempted.
Keywords: EGFR mutation; epidermal growth factor receptor tyrosine kinase inhibitor; erlotinib; non-small-cell lung cancer.
Figures
Similar articles
-
Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations.J Thorac Oncol. 2010 Jul;5(7):1048-53. doi: 10.1097/JTO.0b013e3181dd1386. J Thorac Oncol. 2010. PMID: 20512075 Free PMC article.
-
Clinical impact of switching to a second EGFR-TKI after a severe AE related to a first EGFR-TKI in EGFR-mutated NSCLC.Jpn J Clin Oncol. 2012 Jun;42(6):528-33. doi: 10.1093/jjco/hys042. Epub 2012 Mar 28. Jpn J Clin Oncol. 2012. PMID: 22457323
-
The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations.Clin Cancer Res. 2012 Aug 15;18(16):4406-14. doi: 10.1158/1078-0432.CCR-12-0357. Epub 2012 Jun 25. Clin Cancer Res. 2012. PMID: 22733536 Free PMC article.
-
Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway.Clin Lung Cancer. 2009 Jul;10(4):281-9. doi: 10.3816/CLC.2009.n.039. Clin Lung Cancer. 2009. PMID: 19632948 Free PMC article. Review.
-
Effect of smoking status on progression-free and overall survival in non-small cell lung cancer patients receiving erlotinib or gefitinib: a meta-analysis.J Clin Pharm Ther. 2015 Dec;40(6):661-71. doi: 10.1111/jcpt.12332. Epub 2015 Nov 17. J Clin Pharm Ther. 2015. PMID: 26573867
Cited by
-
Targeted therapies for the treatment of non-small-cell lung cancer: Monoclonal antibodies and biological inhibitors.Hum Vaccin Immunother. 2017 Apr 3;13(4):843-853. doi: 10.1080/21645515.2016.1249551. Epub 2016 Nov 10. Hum Vaccin Immunother. 2017. PMID: 27831000 Free PMC article. Review.
-
Podoplanin enhances lung cancer cell growth in vivo by inducing platelet aggregation.Sci Rep. 2017 Jun 22;7(1):4059. doi: 10.1038/s41598-017-04324-1. Sci Rep. 2017. PMID: 28642617 Free PMC article.
-
Lower limb lymphedema in lung adenocarcinoma: Two case reports.Mol Clin Oncol. 2016 Oct;5(4):478-479. doi: 10.3892/mco.2016.988. Epub 2016 Aug 10. Mol Clin Oncol. 2016. PMID: 27699046 Free PMC article.
-
Molecular targets for the treatment of pancreatic cancer: Clinical and experimental studies.World J Gastroenterol. 2016 Jan 14;22(2):776-89. doi: 10.3748/wjg.v22.i2.776. World J Gastroenterol. 2016. PMID: 26811624 Free PMC article. Review.
-
Theoretical method for evaluation of therapeutic effects and adverse effects of epidermal growth factor receptor tyrosine kinase inhibitors in clinical treatment.Med Oncol. 2017 Sep 8;34(10):178. doi: 10.1007/s12032-017-1036-9. Med Oncol. 2017. PMID: 28887613
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. - PubMed
-
- Ohe Y, Ohashi Y, Kubota K, et al. Randomized Phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18(2):317–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous