Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study
- PMID: 25114635
- PMCID: PMC4110547
- DOI: 10.1186/1556-276X-9-340
Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study
Abstract
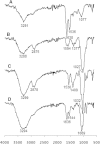
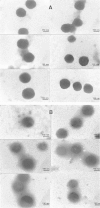
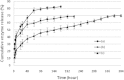
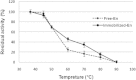
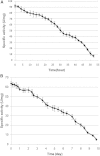
This paper describes the production, purification, and immobilization of l-asparaginase II (ASNase II) in chitosan nanoparticles (CSNPs). ASNase II is an effective antineoplastic agent, used in the acute lymphoblastic leukemia chemotherapy. Cloned ASNase II gene (ansB) in pAED4 plasmid was transformed into Escherichia coli BL21pLysS (DE3) competent cells and expressed under optimal conditions. The lyophilized enzyme was loaded into CSNPs by ionotropic gelation method. In order to get optimal entrapment efficiency, CSNP preparation, chitosan/tripolyphosphate (CS/TPP) ratio, and protein loading were investigated. ASNase II loading into CSNPs was confirmed by Fourier transform infrared (FTIR) spectroscopy, and morphological observation was carried out by transmission electron microscopy. Three absolute CS/TPP ratios were studied. Entrapment efficiency and loading capacity increased with increasing CS and TPP concentration. The best ratio was applied for obtaining optimal ASNase II-loaded CSNPs with the highest entrapment efficiency. Size, zeta potential, entrapment efficiency, and loading capacity of the optimal ASNase II-CSNPs were 340 ± 12 nm, 21.2 ± 3 mV, 76.2% and 47.6%, respectively. The immobilized enzyme showed an increased in vitro half-life in comparison with the free enzyme. The pH and thermostability of the immobilized enzyme was comparable with the free enzyme. This study leads to a better understanding of how to prepare CSNPs, how to achieve high encapsulation efficiency for a high molecular weight protein, and how to prolong the release of protein from CSNPs. A conceptual understanding of biological responses to ASNase II-loaded CSNPs is needed for the development of novel methods of drug delivery.
Keywords: Cross-linking; Enzyme immobilization; Half-life; Ionotropic gelation; Nanoparticle; Optimization.
Figures



References
-
- Matsushima A, Nishimura H, Ashihara Y, Yokota Y, Inada Y. Modification of E. coli asparaginase with 2,4-bis(O-methoxypolyethylene glycol)-6-chloro-S-triazine(activated PEG2); disappearance of binding ability towards anti-serum and retention of enzymic activity. Chem Lett. 1980;9:773–776.
-
- Uren JR, Hargis BJ, Beardsley P. Immunological and pharmacological characterization of poly-DL-alanyl-modified Erwinia carotovora L-asparaginase. Cancer Res. 1982;9:4068–4071. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

