ATP Synthase β-Chain Overexpression in SR-BI Knockout Mice Increases HDL Uptake and Reduces Plasma HDL Level
- PMID: 25114680
- PMCID: PMC4120797
- DOI: 10.1155/2014/356432
ATP Synthase β-Chain Overexpression in SR-BI Knockout Mice Increases HDL Uptake and Reduces Plasma HDL Level
Abstract
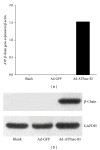
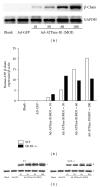
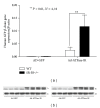
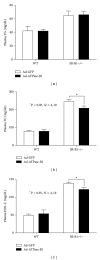
HDL cholesterol is known to be inversely correlated with cardiovascular disease due to its diverse antiatherogenic functions. SR-BI mediates the selective uptake of HDL-C. SR-BI knockout diminishes but does not completely block the transport of HDL; other receptors may be involved. Ectopic ATP synthase β-chain in hepatocytes has been previously characterized as an apoA-I receptor, triggering HDL internalization. This study was undertaken to identify the overexpression of ectopic ATP synthase β-chain on DIL-HDL uptake in primary hepatocytes in vitro and on plasma HDL levels in SR-BI knockout mice. Human ATP synthase β-chain cDNA was delivered to the mouse liver by adenovirus and GFP adenovirus as control. The adenovirus-mediated overexpression of β-chain was identified at both mRNA and protein levels on mice liver and validated by its increasing of DiL-HDL uptake in primary hepatocytes. In response to hepatic overexpression of β-chain, plasma HDL-C levels and cholesterol were reduced in SR-BI knockout mice, compared with the control. The present data suggest that ATP synthase β-chain can serve as the endocytic receptor of HDL, and its overexpression can reduce plasma HDL-C.
Figures



Similar articles
-
MicroRNA-24 aggravates atherosclerosis by inhibiting selective lipid uptake from HDL cholesterol via the post-transcriptional repression of scavenger receptor class B type I.Atherosclerosis. 2018 Mar;270:57-67. doi: 10.1016/j.atherosclerosis.2018.01.045. Epub 2018 Feb 4. Atherosclerosis. 2018. PMID: 29407889
-
Hepatic SR-BI, not endothelial lipase, expression determines biliary cholesterol secretion in mice.J Lipid Res. 2009 Aug;50(8):1571-80. doi: 10.1194/jlr.M800434-JLR200. Epub 2009 Feb 28. J Lipid Res. 2009. PMID: 19252221 Free PMC article.
-
Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse.Arterioscler Thromb Vasc Biol. 2000 Mar;20(3):721-7. doi: 10.1161/01.atv.20.3.721. Arterioscler Thromb Vasc Biol. 2000. PMID: 10712397
-
Mechanisms regulating hepatic SR-BI expression and their impact on HDL metabolism.Atherosclerosis. 2011 Aug;217(2):299-307. doi: 10.1016/j.atherosclerosis.2011.05.036. Epub 2011 Jun 12. Atherosclerosis. 2011. PMID: 21741044 Review.
-
Roles of scavenger receptor BI and APO A-I in selective uptake of HDL cholesterol by adrenal cells.Endocr Res. 2000 Nov;26(4):639-51. doi: 10.3109/07435800009048584. Endocr Res. 2000. PMID: 11196441 Review.
Cited by
-
Scavenger Receptor Class B Type 1 Deletion Led to Coronary Atherosclerosis and Ischemic Heart Disease in Low-density Lipoprotein Receptor Knockout Mice on Modified Western-type Diet.J Atheroscler Thromb. 2017 Feb 1;24(2):133-146. doi: 10.5551/jat.33019. Epub 2016 Jul 2. J Atheroscler Thromb. 2017. PMID: 27373983 Free PMC article.
-
In vitro and in vivo studies of nanoparticles of chitosan-Pandanus tectorius fruit extract as new alternative treatment for hypercholesterolemia via Scavenger Receptor Class B type 1 pathway.Saudi Pharm J. 2020 Oct;28(10):1263-1275. doi: 10.1016/j.jsps.2020.08.017. Epub 2020 Aug 28. Saudi Pharm J. 2020. PMID: 33132720 Free PMC article.
-
Ectopic ATP synthase stimulates the secretion of extracellular vesicles in cancer cells.Commun Biol. 2023 Jun 15;6(1):642. doi: 10.1038/s42003-023-05008-5. Commun Biol. 2023. PMID: 37322056 Free PMC article.
-
ATP Secretion and Metabolism in Regulating Pancreatic Beta Cell Functions and Hepatic Glycolipid Metabolism.Front Physiol. 2022 Jun 21;13:918042. doi: 10.3389/fphys.2022.918042. eCollection 2022. Front Physiol. 2022. PMID: 35800345 Free PMC article. Review.
References
-
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. - PubMed
-
- Brewer HB, Jr., Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(10):1755–1760. - PubMed
-
- Assmann G, Gotto AM., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(23, supplement 1):III8–III14. - PubMed
-
- Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Current Drug Targets. 2008;9(3):196–203. - PubMed
-
- Nofer J, Kehrel B, Fobker M, Levkau B, Assmann G, Eckardstein AV. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161(1):1–16. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

