Brachyury cooperates with Wnt/β-catenin signalling to elicit primitive-streak-like behaviour in differentiating mouse embryonic stem cells
- PMID: 25115237
- PMCID: PMC4171571
- DOI: 10.1186/s12915-014-0063-7
Brachyury cooperates with Wnt/β-catenin signalling to elicit primitive-streak-like behaviour in differentiating mouse embryonic stem cells
Abstract
Background: The formation of the primitive streak is the first visible sign of gastrulation, the process by which the three germ layers are formed from a single epithelium during early development. Embryonic stem cells (ESCs) provide a good system for understanding the molecular and cellular events associated with these processes. Previous work, both in embryos and in culture, has shown how converging signals from both nodal/TGFβR and Wnt/β-catenin signalling pathways specify cells to adopt a primitive-streak-like fate and direct them to undertake an epithelial-to-mesenchymal transition (EMT). However, many of these approaches have relied on genetic analyses without taking into account the temporal progression of events within single cells. In addition, it is still unclear to what extent events in the embryo are able to be reproduced in culture.
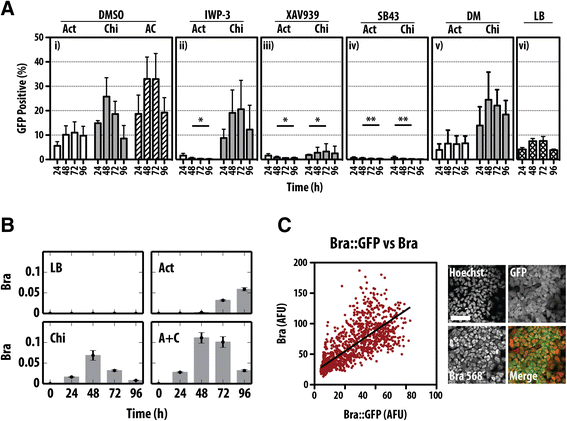
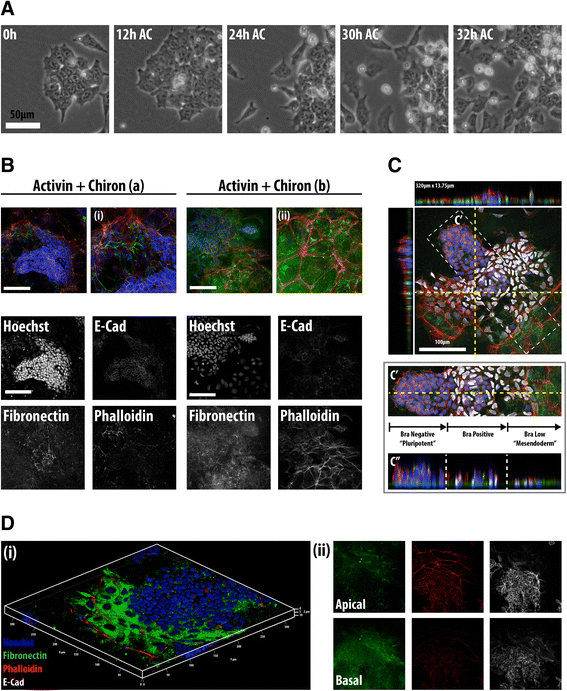
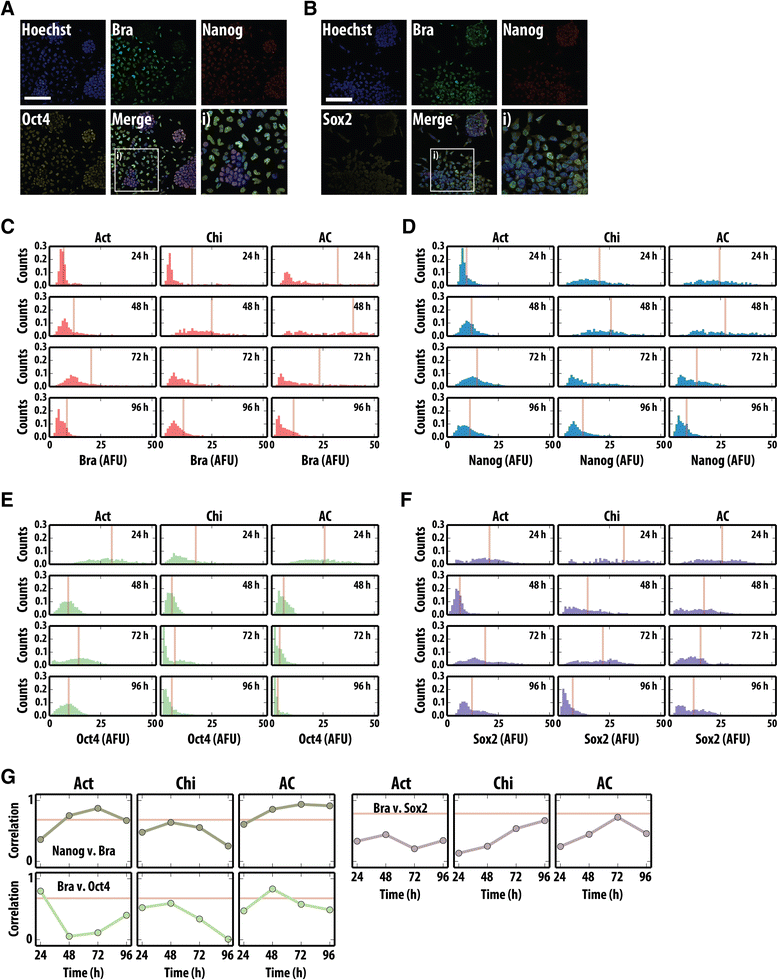
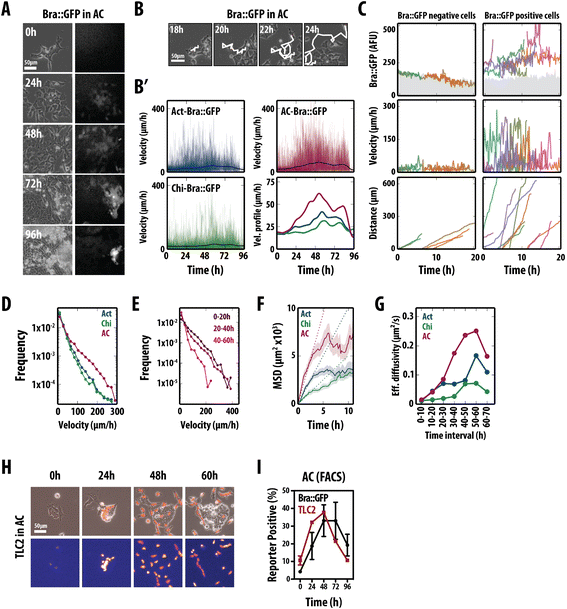
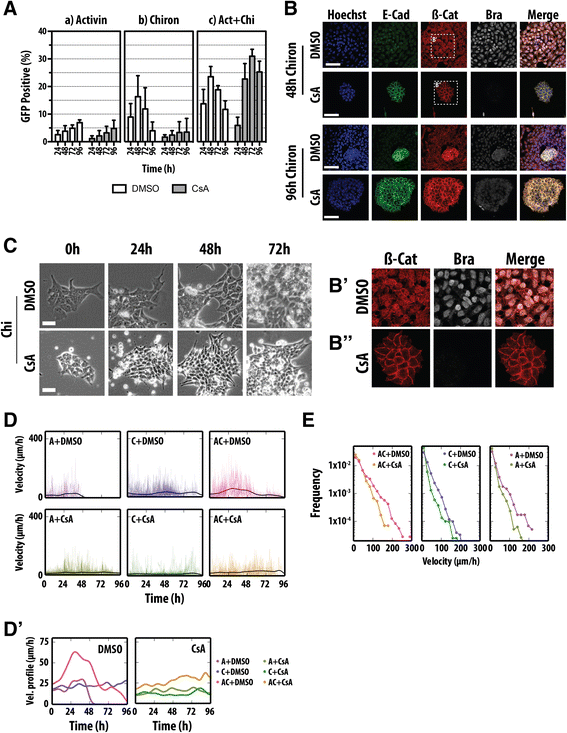
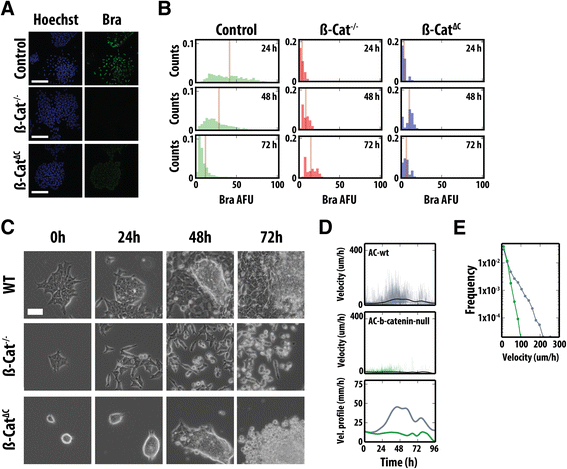
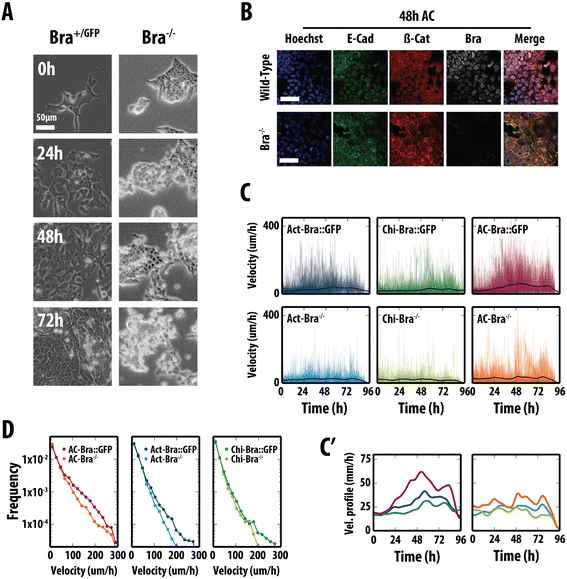
Results: Here, we combine flow cytometry and a quantitative live single-cell imaging approach to demonstrate how the controlled differentiation of mouse ESCs towards a primitive streak fate in culture results in cells displaying many of the characteristics observed during early mouse development including transient brachyury expression, EMT and increased motility. We also find that the EMT initiates the process, and this is both fuelled and terminated by the action of brachyury, whose expression is dependent on the EMT and β-catenin activity.
Conclusions: As a consequence of our analysis, we propose that a major output of brachyury expression is in controlling the velocity of the cells that are transiting out of the primitive streak.
Figures
Similar articles
-
NO-β-catenin crosstalk modulates primitive streak formation prior to embryonic stem cell osteogenic differentiation.J Cell Sci. 2012 Nov 15;125(Pt 22):5564-77. doi: 10.1242/jcs.081703. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946055
-
Basal delamination during mouse gastrulation primes pluripotent cells for differentiation.Dev Cell. 2024 May 20;59(10):1252-1268.e13. doi: 10.1016/j.devcel.2024.03.008. Epub 2024 Apr 4. Dev Cell. 2024. PMID: 38579720 Free PMC article.
-
Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells.PLoS One. 2012;7(3):e33346. doi: 10.1371/journal.pone.0033346. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479388 Free PMC article.
-
A little winning streak: the reptilian-eye view of gastrulation in birds.Dev Growth Differ. 2013 Jan;55(1):52-9. doi: 10.1111/dgd.12014. Epub 2012 Nov 16. Dev Growth Differ. 2013. PMID: 23157408 Review.
-
Wnt signalling in mouse gastrulation and anterior development: new players in the pathway and signal output.Curr Opin Genet Dev. 2013 Aug;23(4):454-60. doi: 10.1016/j.gde.2013.03.001. Epub 2013 Apr 19. Curr Opin Genet Dev. 2013. PMID: 23608663 Review.
Cited by
-
Human ES Cell Culture Conditions Fail to Preserve the Mouse Epiblast State.Stem Cells Int. 2021 Mar 10;2021:8818356. doi: 10.1155/2021/8818356. eCollection 2021. Stem Cells Int. 2021. PMID: 33828592 Free PMC article.
-
Late stage definitive endodermal differentiation can be defined by Daf1 expression.BMC Dev Biol. 2016 May 31;16(1):19. doi: 10.1186/s12861-016-0120-2. BMC Dev Biol. 2016. PMID: 27245320 Free PMC article.
-
β-catenin links cell seeding density to global gene expression during mouse embryonic stem cell differentiation.iScience. 2021 Dec 1;25(1):103541. doi: 10.1016/j.isci.2021.103541. eCollection 2022 Jan 21. iScience. 2021. PMID: 34977504 Free PMC article.
-
β-Catenin-dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):6231-6236. doi: 10.1073/pnas.1713682115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784822 Free PMC article.
-
Divide or Conquer: Cell Cycle Regulation of Invasive Behavior.Trends Cell Biol. 2017 Jan;27(1):12-25. doi: 10.1016/j.tcb.2016.08.003. Epub 2016 Sep 12. Trends Cell Biol. 2017. PMID: 27634432 Free PMC article. Review.
References
-
- Wolpert L, Tickle C. Principles of Development. New York, USA: Oxford University Press; 2010.
-
- Martinez Arias A, Stewart A. Molecular Principles of Animal Development. Oxford, UK: Oxford University Press; 2002.
-
- Gilbert SF. Developmental Biology. Sinauer Associates Inc: Sunderland, Massachusetts, USA; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

