Analysis of the quantitative balance between insulin-like growth factor (IGF)-1 ligand, receptor, and binding protein levels to predict cell sensitivity and therapeutic efficacy
- PMID: 25115504
- PMCID: PMC4236724
- DOI: 10.1186/s12918-014-0098-y
Analysis of the quantitative balance between insulin-like growth factor (IGF)-1 ligand, receptor, and binding protein levels to predict cell sensitivity and therapeutic efficacy
Abstract
Background: The insulin-like growth factor (IGF) system impacts cell proliferation and is highly activated in ovarian cancer. While an attractive therapeutic target, the IGF system is complex with two receptors (IGF1R, IGF2R), two ligands (IGF1, IGF2), and at least six high affinity IGF-binding proteins (IGFBPs) that regulate the bioavailability of IGF ligands. We hypothesized that a quantitative balance between these different network components regulated cell response.
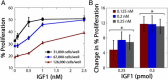
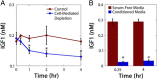
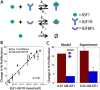
Results: OVCAR5, an immortalized ovarian cancer cell line, were found to be sensitive to IGF1, with the dose of IGF1 (i.e., the total mass of IGF1 available) a more reliable predictor of cell response than ligand concentration. The applied dose of IGF1 was depleted by both cell-secreted IGFBPs and endocytic trafficking, with IGFBPs sequestering up to 90% of the available ligand. To explore how different variables (i.e., IGF1, IGFBPs, and IGF1R levels) impacted cell response, a mass-action steady-state model was developed. Examination of the model revealed that the level of IGF1-IGF1R complexes per cell was directly proportional to the extent of proliferation induced by IGF1. Model analysis suggested, and experimental results confirmed, that IGFBPs present during IGF1 treatment significantly decreased IGF1-mediated proliferation. We utilized this model to assess the efficacy of IGF1 and IGF1R antibodies against different network compositions and determined that IGF1R antibodies were more globally effective due to the receptor-limited state of the network.
Conclusions: Changes that affect IGF1R occupancy have predictable effects on IGF1-induced proliferation and our model captured these effects. Analysis of this model suggests that IGF1R antibodies will be more effective than IGF1 antibodies, although the difference was minimal in conditions with low levels of IGF1 and IGFBPs. Examining how different components of the IGF system influence cell response will be critical to improve our understanding of the IGF signaling network in ovarian cancer.
Figures

References
-
- Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. - PubMed
-
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. - PubMed
-
- Resnicoff M, Abraham D, Yutanawiboonchai W, Rotman HL, Kajstura J, Rubin R, Zoltick P, Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995;55:2463–2469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

