Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function
- PMID: 25115571
- PMCID: PMC4526211
- DOI: 10.1111/1440-1681.12285
Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function
Abstract
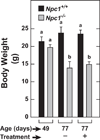
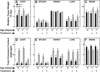
In Niemann-Pick type C (NPC) disease, loss-of-function mutations in either NPC1 or NPC2 result in progressive accumulation of unesterified cholesterol (UC) and glycosphingolipids in all organs, leading to neurodegeneration, pulmonary dysfunction and sometimes liver failure. There is no cure for this disorder. Studies using primarily NPC mouse models have shown that systemic administration of 2-hydroxypropyl-β-cyclodextrin (2HPβCD), starting in early neonatal life, diminishes UC accumulation in most organs, slows disease progression and extends lifespan. The key question now is whether delaying the start of 2HPβCD treatment until early adulthood, when the amount of entrapped UC throughout the body is markedly elevated, has any of the benefits found when treatment begins at 7 days of age. In the present study, Npc1(-/-) and Npc1(+/+) mice were given saline or 2HPβCD subcutaneously at 49, 56, 63 and 70 days of age, with measurements of organ weights, liver function tests and tissue cholesterol levels performed at 77 days. In Npc1(-/-) mice, treatment with 2HPβCD from 49 days reduced whole-liver cholesterol content at 77 days from 33.0 ± 1.0 to 9.1 ± 0.5 mg/organ. Comparable improvements were seen in other organs, such as the spleen, and in the animal as a whole. There was a transient increase in biliary cholesterol concentration in Npc1(-/-) mice after 2HPβCD. Plasma alanine aminotransferase and aspartate aminotransferase activities in 77-day-old 2HPβCD-treated Npc1(-/-) mice were reduced compared with saline-treated controls. The lifespan of Npc1(-/-) mice given 2HPβCD marginally exceeded that of the saline-treated controls (99 ± 1.1 vs 94 ± 1.4 days, respectively; P < 0.05). Thus, 2HPβCD is effective in mobilizing entrapped cholesterol in late-stage NPC disease leading to improved liver function.
Keywords: biliary cholesterol; brain; hepatosplenomegaly; lifespan; lung; lysosomal storage disease; relative organ weight.
© 2014 Wiley Publishing Asia Pty Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
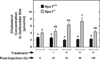


References
-
- Erickson RP. Current controversies in Niemann-Pick C1 disease: steroids or gangliosides; neurons or neurons and glia. J Appl Genet. 2013;54:215–224. - PubMed
-
- Helquist P, Wiest O. Current status of drug therapy development for Niemann-Pick type C disease. Drugs Future. 2009;34:315–331.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

