SdrC induces staphylococcal biofilm formation through a homophilic interaction
- PMID: 25115812
- PMCID: PMC5718044
- DOI: 10.1111/mmi.12750
SdrC induces staphylococcal biofilm formation through a homophilic interaction
Abstract
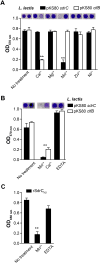
The molecular pathogenesis of many Staphylococcus aureus infections involves growth of bacteria as biofilm. In addition to polysaccharide intercellular adhesin (PIA) and extracellular DNA, surface proteins appear to mediate the transition of bacteria from planktonic growth to sessile lifestyle as well as biofilm growth, and can enable these processes even in the absence of PIA expression. However, the molecular mechanisms by which surface proteins contribute to biofilm formation are incompletely understood. Here we demonstrate that self-association of the serine-aspartate repeat protein SdrC promotes both bacterial adherence to surfaces and biofilm formation. However, this homophilic interaction is not required for the attachment of bacteria to abiotic surfaces. We identified the subdomain that mediates SdrC dimerization and subsequent cell-cell interactions. In addition, we determined that two adjacently located amino acid sequences within this subdomain are required for the SdrC homophilic interaction. Comparative amino acid sequence analysis indicated that these binding sites are conserved. In summary, our study identifies SdrC as a novel molecular determinant in staphylococcal biofilm formation and describes the mechanism responsible for intercellular interactions. Furthermore, these findings contribute to a growing body of evidence suggesting that homophilic interactions between surface proteins present on neighbouring bacteria induce biofilm growth.
© 2014 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3738-3743. doi: 10.1073/pnas.1616805114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320940 Free PMC article.
-
Protein-based biofilm matrices in Staphylococci.Front Cell Infect Microbiol. 2014 Dec 10;4:171. doi: 10.3389/fcimb.2014.00171. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25540773 Free PMC article. Review.
-
Genetic and Biochemical Analysis of CodY-Mediated Cell Aggregation in Staphylococcus aureus Reveals an Interaction between Extracellular DNA and Polysaccharide in the Extracellular Matrix.J Bacteriol. 2020 Mar 26;202(8):e00593-19. doi: 10.1128/JB.00593-19. Print 2020 Mar 26. J Bacteriol. 2020. PMID: 32015143 Free PMC article.
-
Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects.Front Cell Infect Microbiol. 2015 Feb 10;5:7. doi: 10.3389/fcimb.2015.00007. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25713785 Free PMC article. Review.
-
Structural Basis of Staphylococcus aureus Surface Protein SdrC.Biochemistry. 2020 Apr 21;59(15):1465-1469. doi: 10.1021/acs.biochem.0c00124. Epub 2020 Apr 7. Biochemistry. 2020. PMID: 32250096
Cited by
-
Biofilm Formation of Multidrug-Resistant MRSA Strains Isolated from Different Types of Human Infections.Pathogens. 2021 Jul 30;10(8):970. doi: 10.3390/pathogens10080970. Pathogens. 2021. PMID: 34451434 Free PMC article.
-
Role of Lactobacillus in Female Infertility Via Modulating Sperm Agglutination and Immobilization.Front Cell Infect Microbiol. 2021 Jan 25;10:620529. doi: 10.3389/fcimb.2020.620529. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33569356 Free PMC article. Review.
-
Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens.Front Microbiol. 2015 Aug 20;6:841. doi: 10.3389/fmicb.2015.00841. eCollection 2015. Front Microbiol. 2015. PMID: 26347727 Free PMC article. Review.
-
The Role of Staphylococcal Biofilm on the Surface of Implants in Orthopedic Infection.Microorganisms. 2022 Sep 26;10(10):1909. doi: 10.3390/microorganisms10101909. Microorganisms. 2022. PMID: 36296183 Free PMC article. Review.
-
Identification of Three Clf-Sdr Subfamily Proteins in Staphylococcus warneri, and Comparative Genomics Analysis of a Locus Encoding CWA Proteins in Staphylococcus Species.Front Microbiol. 2021 Jul 29;12:691087. doi: 10.3389/fmicb.2021.691087. eCollection 2021. Front Microbiol. 2021. PMID: 34394031 Free PMC article.
References
-
- Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, Engelke G, et al. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. FEBS. 1992;204:1149–1154. - PubMed
-
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

