Src family kinases: at the forefront of platelet activation
- PMID: 25115887
- PMCID: PMC4186533
- DOI: 10.1182/blood-2014-01-453134
Src family kinases: at the forefront of platelet activation
Abstract
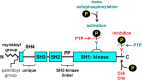
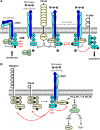
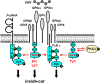
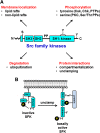
Src family kinases (SFKs) play a central role in mediating the rapid response of platelets to vascular injury. They transmit activation signals from a diverse repertoire of platelet surface receptors, including the integrin αIIbβ3, the immunoreceptor tyrosine-based activation motif-containing collagen receptor complex GPVI-FcR γ-chain, and the von Willebrand factor receptor complex GPIb-IX-V, which are essential for thrombus growth and stability. Ligand-mediated clustering of these receptors triggers an increase in SFK activity and downstream tyrosine phosphorylation of enzymes, adaptors, and cytoskeletal proteins that collectively propagate the signal and coordinate platelet activation. A growing body of evidence has established that SFKs also contribute to Gq- and Gi-coupled receptor signaling that synergizes with primary activation signals to maximally activate platelets and render them prothrombotic. Interestingly, SFKs concomitantly activate inhibitory pathways that limit platelet activation and thrombus size. In this review, we discuss past discoveries that laid the foundation for this fundamental area of platelet signal transduction, recent progress in our understanding of the distinct and overlapping functions of SFKs in platelets, and new avenues of research into mechanisms of SFK regulation. We also highlight the thrombotic and hemostatic consequences of targeting platelet SFKs.
© 2014 by The American Society of Hematology.
Figures




References
-
- Ozaki Y, Asazuma N, Suzuki-Inoue K, Berndt MC. Platelet GPIb-IX-V-dependent signaling. J Thromb Haemost. 2005;3(8):1745–1751. - PubMed
-
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb β3 signaling in platelets. J Thromb Haemost. 2005;3(8):1752–1762. - PubMed
-
- Nuyttens BP, Thijs T, Deckmyn H, Broos K. Platelet adhesion to collagen. Thromb Res. 2011;127(Suppl 2):S26–S29. - PubMed
-
- Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

