Vaniprevir plus peginterferon alfa-2a and ribavirin in treatment-experienced Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase II study
- PMID: 25115901
- PMCID: PMC4318982
- DOI: 10.1007/s00535-014-0979-2
Vaniprevir plus peginterferon alfa-2a and ribavirin in treatment-experienced Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase II study
Abstract
Background: Vaniprevir (MK-7009) is a hepatitis C virus (HCV) non-structural 3/4a protease inhibitor which significantly increases virologic response rates in HCV genotype (GT) 1-infected patients when added to peginterferon and ribavirin (PR).
Methods: This was a phase II, multicenter, double-blind, randomized, dose-ranging study in Japanese patients with HCV GT1 infection and previous relapse. Patients received twice daily vaniprevir 100, 300, or 600 mg, or placebo plus PR for 4 weeks then PR alone for 2 weeks. Further treatment with PR was continued up to a maximum of 72 weeks. The primary endpoint was rapid virologic response (RVR; undetectable HCV RNA at treatment week 4).
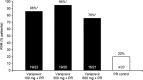
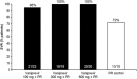
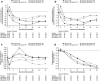
Results: Ninety patients completed 4 weeks of vaniprevir/placebo plus PR. Rates of RVR were significantly higher with vaniprevir compared with placebo (86, 95, and 76 % in the vaniprevir 100-, 300-, and 600-mg arms versus 20 % with control; p<0.001 for all comparisons). Rates of SVR, an exploratory analysis, in the vaniprevir 100-, 300-, 600-mg, and control arms were 95, 100, 100, and 72 %, respectively. No patient had virologic breakthrough or non-response while receiving vaniprevir. There were no serious adverse events (AEs) or discontinuations due to an AE during vaniprevir treatment. Diarrhea and nausea were more common with vaniprevir 600 mg than control or lower vaniprevir doses.
Conclusion: The addition of vaniprevir to PR was associated with an increase in RVR and SVR. Combined with a generally safe and well-tolerated profile, these data supported the further evaluation of vaniprevir in Japanese patients with HCV GT1 infection (#NCT00880763).
Figures

Similar articles
-
Vaniprevir plus peginterferon alfa-2b and ribavirin in treatment-experienced Japanese patients with hepatitis C virus genotype 1 (GT1b) infection: Phase 3 studies.J Gastroenterol Hepatol. 2016 Oct;31(10):1674-1683. doi: 10.1111/jgh.13328. J Gastroenterol Hepatol. 2016. PMID: 26936417 Clinical Trial.
-
Vaniprevir plus peginterferon alfa-2b and ribavirin in treatment-naive Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase III study.J Gastroenterol. 2016 Apr;51(4):390-403. doi: 10.1007/s00535-015-1120-x. Epub 2015 Sep 25. J Gastroenterol. 2016. PMID: 26403160 Free PMC article. Clinical Trial.
-
Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment-experienced patients with chronic HCV genotype 1 infection and cirrhosis.Clin Gastroenterol Hepatol. 2014 Jun;12(6):1029-37.e5. doi: 10.1016/j.cgh.2013.09.067. Epub 2013 Oct 10. Clin Gastroenterol Hepatol. 2014. PMID: 24120953 Clinical Trial.
-
Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-naïve patients with chronic hepatitis C: a randomized phase II study.Hepatology. 2012 Sep;56(3):884-93. doi: 10.1002/hep.25743. Epub 2012 Jul 17. Hepatology. 2012. PMID: 22473713 Clinical Trial.
-
Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C.Drugs. 2012 Mar 26;72(5):619-41. doi: 10.2165/11208370-000000000-00000. Drugs. 2012. PMID: 22439668 Review.
Cited by
-
Daclatasvir and asunaprevir combination therapy for patients with chronic hepatitis C virus genotype 1b infection in real world.Korean J Intern Med. 2019 Jul;34(4):794-801. doi: 10.3904/kjim.2017.368. Epub 2018 May 25. Korean J Intern Med. 2019. PMID: 29792020 Free PMC article.
-
New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials.J Clin Exp Hepatol. 2019 Jul-Aug;9(4):522-538. doi: 10.1016/j.jceh.2018.07.004. Epub 2018 Jul 19. J Clin Exp Hepatol. 2019. PMID: 31516269 Free PMC article. Review.
-
Factors associated with the response to interferon-based antiviral therapies for chronic hepatitis C.World J Hepatol. 2015 Nov 18;7(26):2681-7. doi: 10.4254/wjh.v7.i26.2681. World J Hepatol. 2015. PMID: 26609345 Free PMC article. Review.
-
The clinical features of patients with a Y93H variant of hepatitis C virus detected by a PCR invader assay.J Gastroenterol. 2016 Jan;51(1):63-70. doi: 10.1007/s00535-015-1080-1. Epub 2015 Apr 24. J Gastroenterol. 2016. PMID: 25904097
-
New resistance-associated substitutions and failure of dual oral therapy with daclatasvir and asunaprevir.J Gastroenterol. 2017 Jul;52(7):855-867. doi: 10.1007/s00535-016-1303-0. Epub 2017 Jan 11. J Gastroenterol. 2017. PMID: 28078469
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

