Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage
- PMID: 25115999
- PMCID: PMC4130603
- DOI: 10.1371/journal.pone.0104697
Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage
Abstract
Objective: To investigate grey (GM) and white matter (WM) abnormalities and their effects on cognitive and behavioral deficits in a large, phenotypically and genotypically well-characterized cohort of classic adult (aDM1, age at onset ≥ 20 years) or juvenile (jDM1, age at onset <20 years) patients with myotonic dystrophy type 1 (DM1).
Methods: A case-control study including 51 DM1 patients (17 jDM1 and 34 aDM1) and 34 controls was conducted at an academic medical center. Clinical, cognitive and structural MRI evaluations were obtained. Quantitative assessments of regional GM volumes, WM hyperintensities (WMHs), and microstructural WM tract damage were performed. The association between structural brain damage and clinical and cognitive findings was assessed.
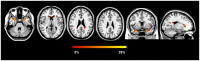
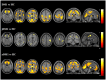
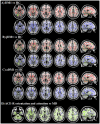
Results: DM1 patients showed a high prevalence of WMHs, severe regional GM atrophy including the key nodes of the sensorimotor and main cognitive brain networks, and WM microstructural damage of the interhemispheric, corticospinal, limbic and associative pathways. WM tract damage extends well beyond the focal WMHs. While aDM1 patients had severe patterns of GM atrophy and WM tract damage, in jDM1 patients WM abnormalities exceeded GM involvement. In DM1, WMHs and microstructural damage, but not GM atrophy, correlated with cognitive deficits.
Conclusions: WM damage, through a disconnection between GM structures, is likely to be the major contributor to cognitive impairment in DM1. Our MRI findings in aDM1 and jDM1 patients support the hypothesis of a degenerative (premature aging) origin of the GM abnormalities and of developmental changes as the principal substrates of microstructural WM alterations in DM1.
Conflict of interest statement
Figures
References
-
- Turner C, Hilton-Jones D (2010) The myotonic dystrophies: diagnosis and management. Journal of neurology, neurosurgery, and psychiatry 81: 358–367. - PubMed
-
- Meola G, Sansone V (2007) Cerebral involvement in myotonic dystrophies. Muscle & nerve 36: 294–306. - PubMed
-
- Vermersch P, Sergeant N, Ruchoux MM, Hofmann-Radvanyi H, Wattez A, et al. (1996) Specific tau variants in the brains of patients with myotonic dystrophy. Neurology 47: 711–717. - PubMed
-
- Sergeant N, Sablonniere B, Schraen-Maschke S, Ghestem A, Maurage CA, et al. (2001) Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Human molecular genetics 10: 2143–2155. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

