Differential and conditional activation of PKC-isoforms dictates cardiac adaptation during physiological to pathological hypertrophy
- PMID: 25116170
- PMCID: PMC4130596
- DOI: 10.1371/journal.pone.0104711
Differential and conditional activation of PKC-isoforms dictates cardiac adaptation during physiological to pathological hypertrophy
Abstract
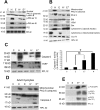
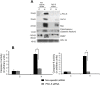
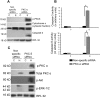
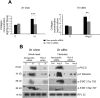
A cardiac hypertrophy is defined as an increase in heart mass which may either be beneficial (physiological hypertrophy) or detrimental (pathological hypertrophy). This study was undertaken to establish the role of different protein kinase-C (PKC) isoforms in the regulation of cardiac adaptation during two types of cardiac hypertrophy. Phosphorylation of specific PKC-isoforms and expression of their downstream proteins were studied during physiological and pathological hypertrophy in 24 week male Balb/c mice (Mus musculus) models, by reverse transcriptase-PCR, western blot analysis and M-mode echocardiography for cardiac function analysis. PKC-δ was significantly induced during pathological hypertrophy while PKC-α was exclusively activated during physiological hypertrophy in our study. PKC-δ activation during pathological hypertrophy resulted in cardiomyocyte apoptosis leading to compromised cardiac function and on the other hand, activation of PKC-α during physiological hypertrophy promoted cardiomyocyte growth but down regulated cellular apoptotic load resulting in improved cardiac function. Reversal in PKC-isoform with induced activation of PKC-δ and simultaneous inhibition of phospho-PKC-α resulted in an efficient myocardium to deteriorate considerably resulting in compromised cardiac function during physiological hypertrophy via augmentation of apoptotic and fibrotic load. This is the first report where PKC-α and -δ have been shown to play crucial role in cardiac adaptation during physiological and pathological hypertrophy respectively thereby rendering compromised cardiac function to an otherwise efficient heart by conditional reversal of their activation.
Conflict of interest statement
Figures



Similar articles
-
Cardiomyocyte targeted overexpression of IGF1 during detraining restores compromised cardiac condition via mTORC2 mediated switching of PKCδ to PKCα.Biochim Biophys Acta Mol Basis Dis. 2019 Oct 1;1865(10):2736-2752. doi: 10.1016/j.bbadis.2019.07.003. Epub 2019 Jul 9. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31299218
-
The alpha(1)-adrenoceptor subtype- and protein kinase C isoform-dependence of Norepinephrine's actions in cardiomyocytes.J Mol Cell Cardiol. 2000 Jul;32(7):1193-209. doi: 10.1006/jmcc.2000.1153. J Mol Cell Cardiol. 2000. PMID: 10860763
-
Role of protein kinase C in caerulein induced expression of substance P and neurokinin-1-receptors in murine pancreatic acinar cells.J Cell Mol Med. 2011 Oct;15(10):2139-49. doi: 10.1111/j.1582-4934.2010.01205.x. J Cell Mol Med. 2011. PMID: 20973912 Free PMC article.
-
PKC and PKN in heart disease.J Mol Cell Cardiol. 2019 Mar;128:212-226. doi: 10.1016/j.yjmcc.2019.01.029. Epub 2019 Feb 8. J Mol Cell Cardiol. 2019. PMID: 30742812 Free PMC article. Review.
-
Role and Mechanism of PKC-δ for Cardiovascular Disease: Current Status and Perspective.Front Cardiovasc Med. 2022 Feb 15;9:816369. doi: 10.3389/fcvm.2022.816369. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35242825 Free PMC article. Review.
Cited by
-
Protein kinase C isozyme expression in right ventricular hypertrophy induced by pulmonary hypertension in chronically hypoxic rats.Mol Med Rep. 2017 Oct;16(4):3833-3840. doi: 10.3892/mmr.2017.7098. Epub 2017 Jul 27. Mol Med Rep. 2017. PMID: 28765942 Free PMC article.
-
Quantitative analysis of ATM phosphorylation in lymphocytes.DNA Repair (Amst). 2019 Aug;80:1-7. doi: 10.1016/j.dnarep.2019.06.002. Epub 2019 Jun 4. DNA Repair (Amst). 2019. PMID: 31176958 Free PMC article.
-
Exercise-induced signaling pathways to counteracting cardiac apoptotic processes.Front Cell Dev Biol. 2022 Aug 11;10:950927. doi: 10.3389/fcell.2022.950927. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036015 Free PMC article. Review.
-
Protein Kinase C Alpha Cellular Distribution, Activity, and Proximity with Lamin A/C in Striated Muscle Laminopathies.Cells. 2020 Oct 31;9(11):2388. doi: 10.3390/cells9112388. Cells. 2020. PMID: 33142761 Free PMC article.
-
Pharmacological Protein Kinase C Modulators Reveal a Pro-hypertrophic Role for Novel Protein Kinase C Isoforms in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes.Front Pharmacol. 2021 Jan 20;11:553852. doi: 10.3389/fphar.2020.553852. eCollection 2020. Front Pharmacol. 2021. PMID: 33584253 Free PMC article.
References
-
- Catalucci D, Latronico MV, Ellingsen O, Condorelli G (2008) Physiological myocardial hypertrophy: how and why? Front Biosci 1: 312–324. - PubMed
-
- Fagard RH (1997) Impact of different sports and training on cardiac structure and function. Cardiol Clin 15: 397–412. - PubMed
-
- Jin H, Yang R, Li W, Lu H, Ryan AM, et al. (2000) Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: 2994–3002. - PubMed
-
- Seidman JG, Seidman C (2001) The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104: 557–567. - PubMed
-
- Weeks KL, McMullen JR (2011) The athlete’s heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology 26: 97–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

