Gait analysis and the cumulative gait index (CGI): Translational tools to assess impairments exhibited by rats with olivocerebellar ataxia
- PMID: 25116252
- PMCID: PMC4179979
- DOI: 10.1016/j.bbr.2014.08.004
Gait analysis and the cumulative gait index (CGI): Translational tools to assess impairments exhibited by rats with olivocerebellar ataxia
Abstract
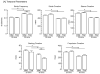
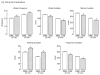
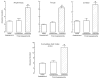
Deviations from 'normal' locomotion exhibited by humans and laboratory animals may be determined using automated systems that capture both temporal and spatial gait parameters. Although many measures generated by these systems are unrelated and independent, some may be related and dependent, representing redundant assessments of function. To investigate this possibility, a treadmill-based system was used to capture gait parameters from normal and ataxic rats, and a multivariate analysis was conducted to determine deviations from normal. Rats were trained on the treadmill at two speeds, and gait parameters were generated prior to and following lesions of the olivocerebellar pathway. Control (non-lesioned) animals exhibited stable hindlimb gait parameters across assessments at each speed. Lesioned animals exhibited alterations in multiple hindlimb gait parameters, characterized by significant increases in stride frequency, braking duration, stance width, step angle, and paw angle and decreases in stride, stance, swing and propulsion durations, stride length and paw area. A principal component analysis of initial hindlimb measures indicated three uncorrelated factors mediating performance, termed Rhythmicity, Thrust and Contact. Deviation in the performance of each animal from the group mean was determined for each factor and values summed to yield the cumulative gait index (CGI), a single value reflecting variation within the group. The CGI for lesioned animals increased 2.3-fold relative to unlesioned animals. This study characterizes gait alterations in laboratory rats rendered ataxic by destruction of the climbing fiber pathway innervating Purkinje cells and demonstrates that a single index can be used to describe overall gait impairments.
Keywords: Ataxia; DigiGait™ Imaging System; Olivocerebellar-lesioned rat; Treadmill gait analysis.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures





References
-
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–43. - PubMed
-
- Mullenix P, Norton S, Culver B. Locomotor damage in rats after x-irradiation in utero. Exp Neurol. 1975;48:310–24. - PubMed
-
- Clarke KA, Parker AJ. A quantitative study of normal locomotion in the rat. Physiol Behav. 1986;38:345–51. - PubMed
-
- Tang W, Su D. Locomotion analysis and its applications in neurological disorders detection: state-of-art review. Network Modeling Analysis in Health Informatics and Bioinformatics. 2013;2:1–12.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

