Retinal dendritic cell recruitment, but not function, was inhibited in MyD88 and TRIF deficient mice
- PMID: 25116321
- PMCID: PMC4149240
- DOI: 10.1186/s12974-014-0143-1
Retinal dendritic cell recruitment, but not function, was inhibited in MyD88 and TRIF deficient mice
Abstract
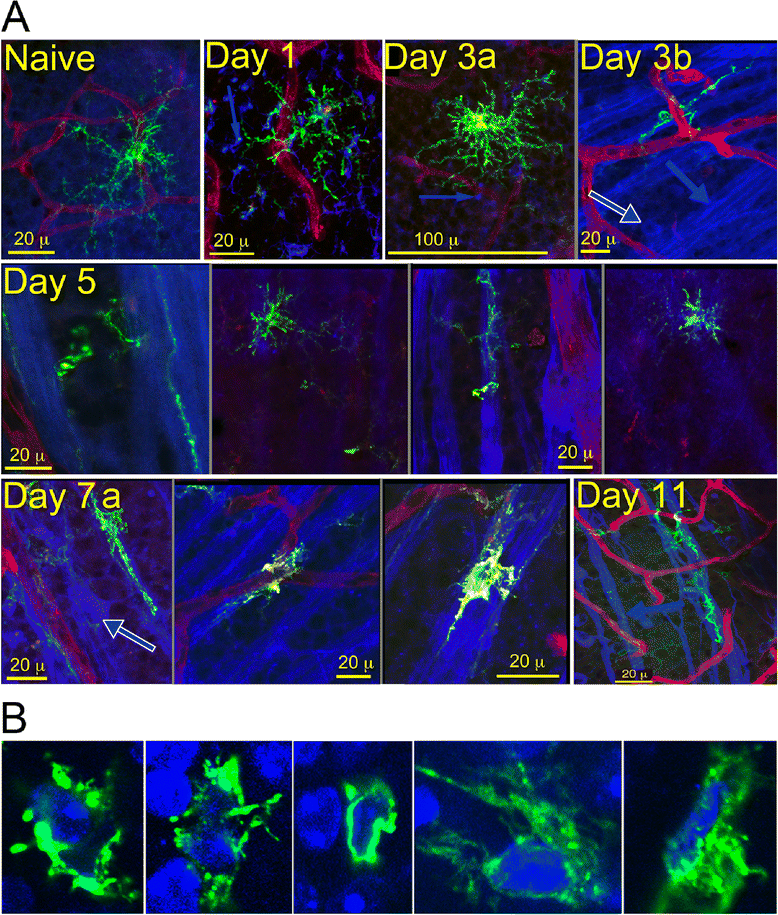
Background: Immune system cells are known to affect loss of neurons due to injury or disease. Recruitment of immune cells following retinal/CNS injury has been shown to affect the health and survival of neurons in several models. We detected close, physical contact between dendritic cells and retinal ganglion cells following an optic nerve crush, and sought to understand the underlying mechanisms.
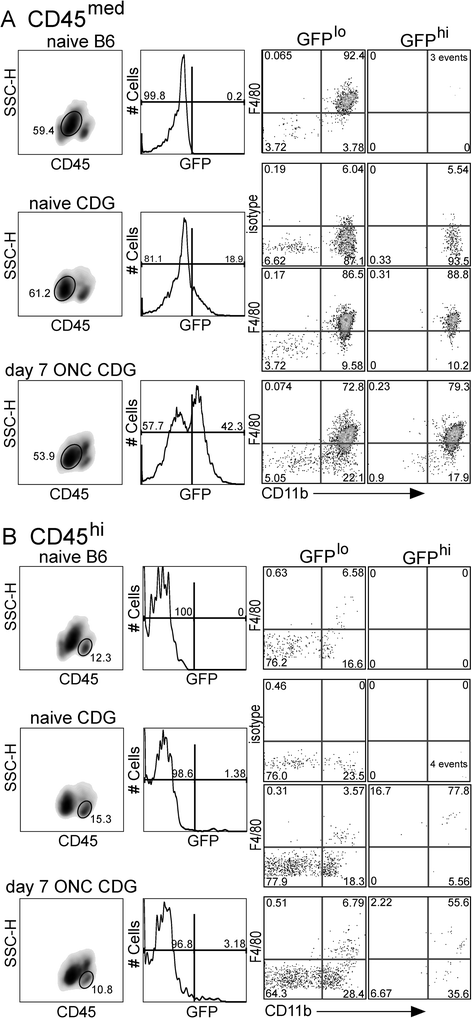
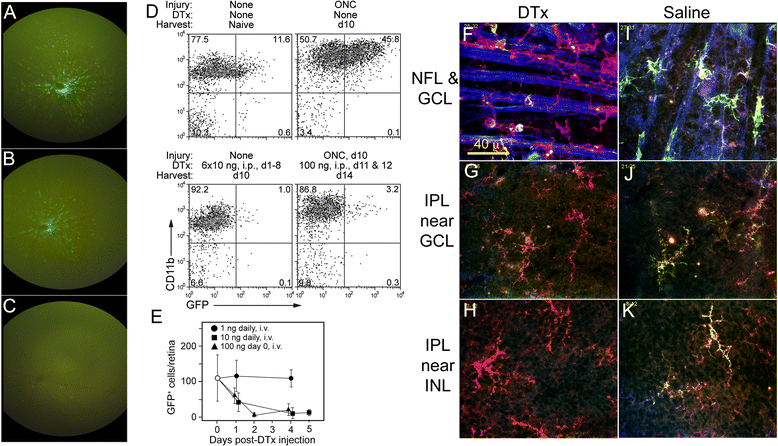
Methods: CD11c-DTR/GFP mice producing a chimeric protein of diphtheria toxin receptor (DTR) and GFP from a transgenic CD11c promoter were used in conjunction with mice deficient in MyD88 and/or TRIF. Retinal ganglion cell injury was induced by an optic nerve crush, and the resulting interactions of the GFPhi cells and retinal ganglion cells were examined.
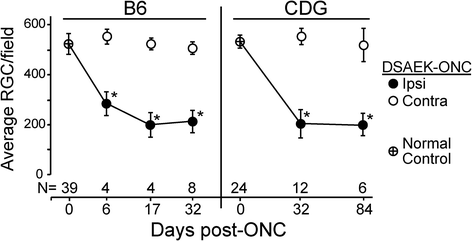
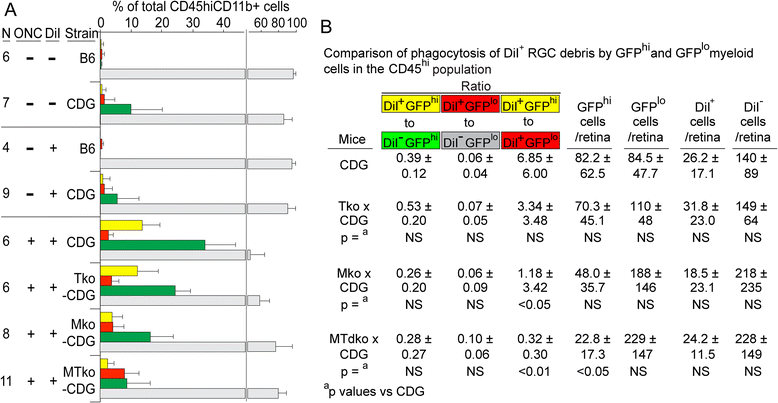
Results: Recruitment of GFPhi dendritic cells to the retina was significantly compromised in MyD88 and TRIF knockout mice. GFPhi dendritic cells played a significant role in clearing fluorescent-labeled retinal ganglion cells post-injury in the CD11c-DTR/GFP mice. In the TRIF and MyD88 deficient mice, the resting level of GFPhi dendritic cells was lower, and their influx was reduced following the optic nerve crush injury. The reduction in GFPhi dendritic cell numbers led to their replacement in the uptake of fluorescent-labeled debris by GFPlo microglia/macrophages. Depletion of GFPhi dendritic cells by treatment with diphtheria toxin also led to their displacement by GFPlo microglia/macrophages, which then assumed close contact with the injured neurons.
Conclusions: The contribution of recruited cells to the injury response was substantial, and regulated by MyD88 and TRIF. However, the presence of these adaptor proteins was not required for interaction with neurons, or the phagocytosis of debris. The data suggested a two-niche model in which resident microglia were maintained at a constant level post-optic nerve crush, while the injury-stimulated recruitment of dendritic cells and macrophages led to their transient appearance in numbers equivalent to or greater than the resident microglia.
Figures




Similar articles
-
Optic nerve as a source of activated retinal microglia post-injury.Acta Neuropathol Commun. 2018 Jul 23;6(1):66. doi: 10.1186/s40478-018-0571-8. Acta Neuropathol Commun. 2018. PMID: 30037353 Free PMC article.
-
The retinal environment induces microglia-like properties in recruited myeloid cells.J Neuroinflammation. 2019 Jul 20;16(1):151. doi: 10.1186/s12974-019-1546-9. J Neuroinflammation. 2019. PMID: 31325968 Free PMC article.
-
Microglial TIR-domain-containing adapter-inducing interferon-β (TRIF) deficiency promotes retinal ganglion cell survival and axon regeneration via nuclear factor-κB.J Neuroinflammation. 2012 Feb 24;9:39. doi: 10.1186/1742-2094-9-39. J Neuroinflammation. 2012. PMID: 22361049 Free PMC article.
-
The TIR-domain-containing adapter inducing interferon-β-dependent signaling cascade plays a crucial role in ischemia-reperfusion-induced retinal injury, whereas the contribution of the myeloid differentiation primary response 88-dependent signaling cascade is not as pivotal.Eur J Neurosci. 2014 Aug;40(3):2502-12. doi: 10.1111/ejn.12603. Epub 2014 Apr 23. Eur J Neurosci. 2014. PMID: 24754835 Free PMC article.
-
[Advances in research on the role of immune cells in optic nerve injury].Zhonghua Yan Ke Za Zhi. 2024 Jan 11;60(1):95-101. doi: 10.3760/cma.j.cn112142-20231012-00132. Zhonghua Yan Ke Za Zhi. 2024. PMID: 38199775 Review. Chinese.
Cited by
-
Parabiosis reveals the correlation between the recruitment of circulating antigen presenting cells to the retina and the induction of spontaneous autoimmune uveoretinitis.J Neuroinflammation. 2022 Dec 9;19(1):295. doi: 10.1186/s12974-022-02660-2. J Neuroinflammation. 2022. PMID: 36494807 Free PMC article.
-
Immunomodulation as a Neuroprotective Strategy for Glaucoma Treatment.Curr Ophthalmol Rep. 2019 Jun;7(2):160-169. Epub 2019 Apr 23. Curr Ophthalmol Rep. 2019. PMID: 31360618 Free PMC article.
-
Optic nerve as a source of activated retinal microglia post-injury.Acta Neuropathol Commun. 2018 Jul 23;6(1):66. doi: 10.1186/s40478-018-0571-8. Acta Neuropathol Commun. 2018. PMID: 30037353 Free PMC article.
-
Tumor necrosis factor alpha has an early protective effect on retinal ganglion cells after optic nerve crush.J Neuroinflammation. 2014 Nov 19;11:194. doi: 10.1186/s12974-014-0194-3. J Neuroinflammation. 2014. PMID: 25407441 Free PMC article.
-
The Role of Retinal Antigen-Presenting Cells in Spontaneous Retinal Autoimmunity.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):26. doi: 10.1167/iovs.66.11.26. Invest Ophthalmol Vis Sci. 2025. PMID: 40787935 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

