A mutation in the CASQ1 gene causes a vacuolar myopathy with accumulation of sarcoplasmic reticulum protein aggregates
- PMID: 25116801
- PMCID: PMC4177304
- DOI: 10.1002/humu.22631
A mutation in the CASQ1 gene causes a vacuolar myopathy with accumulation of sarcoplasmic reticulum protein aggregates
Abstract
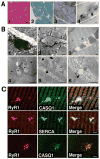
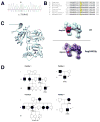
A missense mutation in the calsequestrin-1 gene (CASQ1) was found in a group of patients with a myopathy characterized by weakness, fatigue, and the presence of large vacuoles containing characteristic inclusions resulting from the aggregation of sarcoplasmic reticulum (SR) proteins. The mutation affects a conserved aspartic acid in position 244 (p.Asp244Gly) located in one of the high-affinity Ca(2+) -binding sites of CASQ1 and alters the kinetics of Ca(2+) release in muscle fibers. Expression of the mutated CASQ1 protein in COS-7 cells showed a markedly reduced ability in forming elongated polymers, whereas both in cultured myotubes and in in vivo mouse fibers induced the formation of electron-dense SR vacuoles containing aggregates of the mutant CASQ1 protein that resemble those observed in muscle biopsies of patients. Altogether, these results support the view that a single missense mutation in the CASQ1 gene causes the formation of abnormal SR vacuoles containing aggregates of CASQ1, and other SR proteins, results in altered Ca(2+) release in skeletal muscle fibers, and, hence, is responsible for the clinical phenotype observed in these patients.
Keywords: CASQ1; aggregate myopathy; calsequestrin; sarcoplasmic reticulum; skeletal muscle.
© 2014 WILEY PERIODICALS, INC.
Figures

References
-
- Boncompagni S, d’Amelio L, Fulle S, Fanò G, Protasi F. Progressive disorganization of the excitation-contraction coupling apparatus in ageing human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance. J Gerontol A Biol Sci Med Sci. 2006;61:995–1008. - PubMed
-
- Cho JH, Ko KM, Singaruvelu G, Lee W, Kang GB, Rho SH, Park BJ, Yu JR, Kagawa H, Eom SH, Kim do H, Ann J. Functional importance of polymerization and localization of calsequestrin in C. elegans. J Cell Sci. 2007;120:1551–1558. - PubMed
-
- Damiani E, Volpe P, Margreth A. Coexpression of two isoforms of calsequestrin in rabbit slow-twitch muscle. J Muscle Res Cell Motil. 1990;11:522–530. - PubMed
-
- Doria C, Toniolo L, Verratti V, Cancellara P, Pietrangelo T, Marconi V, Paoli A, Pogliaghi S, Fano G, Reggiani C, Capelli C. Improved VO2 uptake kinetics and shift in muscle fiber type in high altitude trekkers. J Appl Physiol. 2011;111:1597–605. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

