Alterations of the synapse of the inner retinal layers after chronic intraocular pressure elevation in glaucoma animal model
- PMID: 25116810
- PMCID: PMC4237962
- DOI: 10.1186/s13041-014-0053-2
Alterations of the synapse of the inner retinal layers after chronic intraocular pressure elevation in glaucoma animal model
Abstract
Background: Dendrites of retinal ganglion cells (RGCs) synapse with axon terminals of bipolar cells in the inner plexiform layer (IPL). Changes in RGC dendrites and synapses between bipolar cells in the inner retinal layer may critically alter the function of RGCs in glaucoma. Recently, synaptic plasticity has been observed in the adult central nervous system, including the outer retinal layers. However, few studies have focused on changes in the synapses between RGCs and bipolar cells in glaucoma. In the present study, we used a rat model of ocular hypertension induced by episcleral vein cauterization to investigate changes in synaptic structure and protein expression in the inner retinal layer at various time points after moderate intraocular pressure (IOP) elevation.
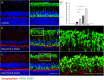
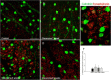
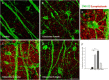
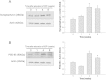
Results: Synaptophysin, a presynaptic vesicle protein, increased throughout the IPL, outer plexiform layer, and outer nuclear layer after IOP elevation. Increased synaptophysin after IOP elevation was expressed in bipolar cells in the innermost IPL. The RGC marker, SMI-32, co-localized with synaptophysin in RGC dendrites and were significantly increased at 1 week and 4 weeks after IOP elevation. Both synaptophysin and postsynaptic vesicle protein, PSD-95, were increased after IOP elevation by western blot analysis. Ribbon synapses in the IPL were quantified and structurally evaluated in retinal sections by transmission electron microscopy. After IOP elevation the total number of ribbon synapses decreased. There were increases in synapse diameter and synaptic vesicle number and decreases in active zone length and the number of docked vesicles after IOP elevation.
Conclusions: Although the total number of synapses decreased as RGCs were lost after IOP elevation, there are attempts to increase synaptic vesicle proteins and immature synapse formation between RGCs and bipolar cells in the inner retinal layers after glaucoma induction.
Figures



Similar articles
-
Selective Vulnerability of Specific Retinal Ganglion Cell Types and Synapses after Transient Ocular Hypertension.J Neurosci. 2016 Aug 31;36(35):9240-52. doi: 10.1523/JNEUROSCI.0940-16.2016. J Neurosci. 2016. PMID: 27581463 Free PMC article.
-
Increased levels of synaptic proteins involved in synaptic plasticity after chronic intraocular pressure elevation and modulation by brain-derived neurotrophic factor in a glaucoma animal model.Dis Model Mech. 2019 Jun 18;12(6):dmm037184. doi: 10.1242/dmm.037184. Dis Model Mech. 2019. PMID: 31142572 Free PMC article.
-
Synaptic degeneration of retinal ganglion cells in a rat ocular hypertension glaucoma model.Cell Mol Neurobiol. 2009 Jun;29(4):575-81. doi: 10.1007/s10571-009-9349-7. Epub 2009 Jan 27. Cell Mol Neurobiol. 2009. PMID: 19172389 Free PMC article.
-
Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas.Prog Retin Eye Res. 2012 Jan;31(1):1-27. doi: 10.1016/j.preteyeres.2011.08.001. Epub 2011 Sep 21. Prog Retin Eye Res. 2012. PMID: 21946033 Review.
-
Influences of Glaucoma on the Structure and Function of Synapses in the Visual System.Antioxid Redox Signal. 2022 Oct;37(10-12):842-861. doi: 10.1089/ars.2021.0253. Epub 2022 Apr 18. Antioxid Redox Signal. 2022. PMID: 35044228 Free PMC article. Review.
Cited by
-
Selective Vulnerability of Specific Retinal Ganglion Cell Types and Synapses after Transient Ocular Hypertension.J Neurosci. 2016 Aug 31;36(35):9240-52. doi: 10.1523/JNEUROSCI.0940-16.2016. J Neurosci. 2016. PMID: 27581463 Free PMC article.
-
Ocular Hypertension Results in Hypoxia within Glia and Neurons throughout the Visual Projection.Antioxidants (Basel). 2022 Apr 29;11(5):888. doi: 10.3390/antiox11050888. Antioxidants (Basel). 2022. PMID: 35624752 Free PMC article.
-
Macroglia-derived thrombospondin 2 regulates alterations of presynaptic proteins of retinal neurons following elevated hydrostatic pressure.PLoS One. 2017 Sep 27;12(9):e0185388. doi: 10.1371/journal.pone.0185388. eCollection 2017. PLoS One. 2017. PMID: 28953973 Free PMC article.
-
Sodium Hyaluronate-Induced Ocular Hypertension in Rats Damages the Direction-Selective Circuit and Inner/Outer Retinal Plexiform Layers.Invest Ophthalmol Vis Sci. 2022 May 2;63(5):2. doi: 10.1167/iovs.63.5.2. Invest Ophthalmol Vis Sci. 2022. PMID: 35503230 Free PMC article.
-
Digital reconstruction and quantitative morphometric analysis of bipolar cells in live rat retinal slices.J Comp Neurol. 2022 Jul;530(10):1700-1728. doi: 10.1002/cne.25308. Epub 2022 Mar 21. J Comp Neurol. 2022. PMID: 35152437 Free PMC article.
References
-
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

