Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: data from two clinical trials in pancreatic cancer
- PMID: 25116885
- PMCID: PMC4190529
- DOI: 10.3390/pharmaceutics6030447
Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: data from two clinical trials in pancreatic cancer
Abstract
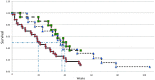
Despite progress in the treatment of pancreatic cancer, there is still a need for improved therapies. In this manuscript, we report clinical experience with a new therapy for the treatment of pancreatic cancer involving the implantation of encapsulated cells over-expressing a cytochrome P450 enzyme followed by subsequent low-dose ifosfamide administrations as a means to target activated ifosfamide to the tumor. The safety and efficacy of the angiographic instillation of encapsulated allogeneic cells overexpressing cytochrome P450 in combination with low-dose systemic ifosfamide administration has now been evaluated in 27 patients in total. These patients were successfully treated in four centers by three different interventional radiologists, arguing strongly that the treatment can be successfully used in different centers. The safety of the intra-arterial delivery of the capsules and the lack of evidence that the patients developed an inflammatory or immune response to the encapsulated cells or encapsulation material was shown in all 27 patients. The ifosfamide dose of 1 g/m2/day used in the first trial was well tolerated by all patients. In contrast, the ifosfamide dose of 2 g/m2/day used in the second trial was poorly tolerated in most patients. Since the median survival in the first trial was 40 weeks and only 33 weeks in the second trial, this strongly suggests that there is no survival benefit to increasing the dose of ifosfamide, and indeed, a lower dose is beneficial for quality of life and the lack of side effects. This is supported by the one-year survival rate in the first trial being 38%, whilst that in the second trial was only 23%. However, taking the data from both trials together, a total of nine of the 27 patients were alive after one year, and two of these nine patients were alive for two years or more.
Figures
Similar articles
-
Long-Term Survival of Cellulose Sulphate-Encapsulated Cells and Metronomic Ifosfamide Control Tumour Growth in Pancreatic Cancer Models-A Prelude to Treating Solid Tumours Effectively in Pets and Humans.Life (Basel). 2023 Dec 18;13(12):2357. doi: 10.3390/life13122357. Life (Basel). 2023. PMID: 38137959 Free PMC article.
-
Use of cell therapy as a means of targeting chemotherapy to inoperable pancreatic cancer.Acta Biochim Pol. 2005;52(3):601-7. Acta Biochim Pol. 2005. PMID: 16175235 Review.
-
Subsieve-size agarose capsules enclosing ifosfamide-activating cells: a strategy toward chemotherapeutic targeting to tumors.Mol Cancer Ther. 2005 Nov;4(11):1786-90. doi: 10.1158/1535-7163.MCT-05-0227. Mol Cancer Ther. 2005. PMID: 16276000
-
Microencapsulated, CYP2B1-transfected cells activating ifosfamide at the site of the tumor: the magic bullets of the 21st century.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S21-4. doi: 10.1007/s00280-002-0448-0. Epub 2002 Apr 12. Cancer Chemother Pharmacol. 2002. PMID: 12042985 Review.
-
Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: results of a phase I/II clinical trial.J Gastroenterol. 2003 Mar;38 Suppl 15:78-84. J Gastroenterol. 2003. PMID: 12698877 Clinical Trial.
Cited by
-
Long-Term Survival of Cellulose Sulphate-Encapsulated Cells and Metronomic Ifosfamide Control Tumour Growth in Pancreatic Cancer Models-A Prelude to Treating Solid Tumours Effectively in Pets and Humans.Life (Basel). 2023 Dec 18;13(12):2357. doi: 10.3390/life13122357. Life (Basel). 2023. PMID: 38137959 Free PMC article.
-
Multilayer Alginate Microcapsules For Live Cell Microencapsulation; Is There Any Preference For Selecting Cationic Polymers?Iran J Pharm Res. 2021 Spring;20(2):173-182. doi: 10.22037/ijpr.2020.114096.14660. Iran J Pharm Res. 2021. PMID: 34567154 Free PMC article. Review.
-
Monitoring implantable immunoisolation devices with intrinsic fluorescence of genipin.J Biophotonics. 2019 Apr;12(4):e201800170. doi: 10.1002/jbio.201800170. Epub 2018 Sep 25. J Biophotonics. 2019. PMID: 30058289 Free PMC article.
-
Semipermeable Cellulose Beads Allow Selective and Continuous Release of Small Extracellular Vesicles (sEV) From Encapsulated Cells.Front Pharmacol. 2020 May 21;11:679. doi: 10.3389/fphar.2020.00679. eCollection 2020. Front Pharmacol. 2020. PMID: 32528280 Free PMC article.
-
Prodrugs and prodrug-activated systems in gene therapy.Mol Ther. 2021 May 5;29(5):1716-1728. doi: 10.1016/j.ymthe.2021.04.006. Epub 2021 Apr 6. Mol Ther. 2021. PMID: 33831557 Free PMC article. Review.
References
-
- Dabernat S., Lafitte M., Bedel A., de Verneuil H., Moreau-Gaudry F. Gene therapy of pancreatic cancer. J. Genet. Syndr. Gene Ther. 2013;4:138.
-
- Amercian Cancer Society, Cancer Facts & Figures. 2013. [(accessed on 24 March 2014)]. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

