A bioenergetic basis for membrane divergence in archaea and bacteria
- PMID: 25116890
- PMCID: PMC4130499
- DOI: 10.1371/journal.pbio.1001926
A bioenergetic basis for membrane divergence in archaea and bacteria
Erratum in
-
Correction: a bioenergetic basis for membrane divergence in archaea and bacteria.PLoS Biol. 2015 Mar 20;13(3):e1002102. doi: 10.1371/journal.pbio.1002102. eCollection 2015 Mar. PLoS Biol. 2015. PMID: 25794363 Free PMC article. No abstract available.
Abstract
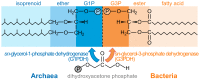
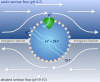
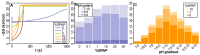
Membrane bioenergetics are universal, yet the phospholipid membranes of archaea and bacteria-the deepest branches in the tree of life-are fundamentally different. This deep divergence in membrane chemistry is reflected in other stark differences between the two domains, including ion pumping and DNA replication. We resolve this paradox by considering the energy requirements of the last universal common ancestor (LUCA). We develop a mathematical model based on the premise that LUCA depended on natural proton gradients. Our analysis shows that such gradients can power carbon and energy metabolism, but only in leaky cells with a proton permeability equivalent to fatty acid vesicles. Membranes with lower permeability (equivalent to modern phospholipids) collapse free-energy availability, precluding exploitation of natural gradients. Pumping protons across leaky membranes offers no advantage, even when permeability is decreased 1,000-fold. We hypothesize that a sodium-proton antiporter (SPAP) provided the first step towards modern membranes. SPAP increases the free energy available from natural proton gradients by ∼60%, enabling survival in 50-fold lower gradients, thereby facilitating ecological spread and divergence. Critically, SPAP also provides a steadily amplifying advantage to proton pumping as membrane permeability falls, for the first time favoring the evolution of ion-tight phospholipid membranes. The phospholipids of archaea and bacteria incorporate different stereoisomers of glycerol phosphate. We conclude that the enzymes involved took these alternatives by chance in independent populations that had already evolved distinct ion pumps. Our model offers a quantitatively robust explanation for why membrane bioenergetics are universal, yet ion pumps and phospholipid membranes arose later and independently in separate populations. Our findings elucidate the paradox that archaea and bacteria share DNA transcription, ribosomal translation, and ATP synthase, yet differ in equally fundamental traits that depend on the membrane, including DNA replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The origin of membrane bioenergetics.Cell. 2012 Dec 21;151(7):1406-16. doi: 10.1016/j.cell.2012.11.050. Cell. 2012. PMID: 23260134
-
Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea.Mol Microbiol. 1995 Dec;18(5):925-32. doi: 10.1111/j.1365-2958.1995.18050925.x. Mol Microbiol. 1995. PMID: 8825096
-
A leaky membrane and a sodium transporter at life's great divergence.PLoS Biol. 2014 Aug 12;12(8):e1001927. doi: 10.1371/journal.pbio.1001927. eCollection 2014 Aug. PLoS Biol. 2014. PMID: 25116919 Free PMC article. No abstract available.
-
Bioenergetic aspects of archaeal and bacterial hydrogen metabolism.Adv Microb Physiol. 2019;74:487-514. doi: 10.1016/bs.ampbs.2019.02.005. Epub 2019 Feb 28. Adv Microb Physiol. 2019. PMID: 31126536 Review.
-
The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments.Antonie Van Leeuwenhoek. 2002 Aug;81(1-4):61-72. doi: 10.1023/a:1020573408652. Antonie Van Leeuwenhoek. 2002. PMID: 12448706 Review.
Cited by
-
Structure and Evolution of the Archaeal Lipid Synthesis Enzyme sn-Glycerol-1-phosphate Dehydrogenase.J Biol Chem. 2015 Aug 28;290(35):21690-704. doi: 10.1074/jbc.M115.647461. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175150 Free PMC article.
-
Viruses and cells intertwined since the dawn of evolution.Virol J. 2015 Oct 16;12:169. doi: 10.1186/s12985-015-0400-7. Virol J. 2015. PMID: 26475454 Free PMC article. Review.
-
Early evolution of polyisoprenol biosynthesis and the origin of cell walls.PeerJ. 2016 Oct 26;4:e2626. doi: 10.7717/peerj.2626. eCollection 2016. PeerJ. 2016. PMID: 27812422 Free PMC article.
-
Intrinsic concentration cycles and high ion fluxes in self-assembled precipitate membranes.Interface Focus. 2019 Dec 6;9(6):20190064. doi: 10.1098/rsfs.2019.0064. Epub 2019 Oct 18. Interface Focus. 2019. PMID: 31641435 Free PMC article.
-
An origin-of-life reactor to simulate alkaline hydrothermal vents.J Mol Evol. 2014 Dec;79(5-6):213-27. doi: 10.1007/s00239-014-9658-4. Epub 2014 Nov 27. J Mol Evol. 2014. PMID: 25428684 Free PMC article.
References
-
- Williams TA, Foster PG, Cox CJ, Embley TM (2013) An archaeal origin of eukaryotes supports only two primary domains of life. Nature 504: 231–236. - PubMed
-
- Embley TM, Martin W (2006) Eukaryotic evolution, changes and challenges. Nature 440: 623–630. - PubMed
-
- Lane N, Martin W (2010) The energetics of genome complexity. Nature 467: 929–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

