Three-dimensional in vitro tumor models for cancer research and drug evaluation
- PMID: 25116894
- PMCID: PMC4171250
- DOI: 10.1016/j.biotechadv.2014.07.009
Three-dimensional in vitro tumor models for cancer research and drug evaluation
Abstract
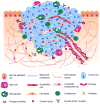
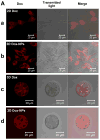
Cancer occurs when cells acquire genomic instability and inflammation, produce abnormal levels of epigenetic factors/proteins and tumor suppressors, reprogram the energy metabolism and evade immune destruction, leading to the disruption of cell cycle/normal growth. An early event in carcinogenesis is loss of polarity and detachment from the natural basement membrane, allowing cells to form distinct three-dimensional (3D) structures that interact with each other and with the surrounding microenvironment. Although valuable information has been accumulated from traditional in vitro studies in which cells are grown on flat and hard plastic surfaces (2D culture), this culture condition does not reflect the essential features of tumor tissues. Further, fundamental understanding of cancer metastasis cannot be obtained readily from 2D studies because they lack the complex and dynamic cell-cell communications and cell-matrix interactions that occur during cancer metastasis. These shortcomings, along with lack of spatial depth and cell connectivity, limit the applicability of 2D cultures to accurate testing of pharmacologically active compounds, free or sequestered in nanoparticles. To recapitulate features of native tumor microenvironments, various biomimetic 3D tumor models have been developed to incorporate cancer and stromal cells, relevant matrix components, and biochemical and biophysical cues, into one spatially and temporally integrated system. In this article, we review recent advances in creating 3D tumor models employing tissue engineering principles. We then evaluate the utilities of these novel models for the testing of anticancer drugs and their delivery systems. We highlight the profound differences in responses from 3D in vitro tumors and conventional monolayer cultures. Overall, strategic integration of biological principles and engineering approaches will both improve understanding of tumor progression and invasion and support discovery of more personalized first line treatments for cancer patients.
Keywords: 3D tumor models; Bioreactors; Cancer therapeutics; Drug delivery; Drug resistance; Hydrogels; Microfluidic devices; Scaffolds.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Becker JL, Souza GR. Using space-based investigations to inform cancer research on Earth. Nat Rev Cancer. 2013;13:315–27. - PubMed
-
- Bourguignon LYW, Singleton PA, Zhu HB, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (Macrophage-Colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–34. - PubMed
-
- Bourguignon LYW, Singleton PA, Zhu HB, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

