A community-based multicenter trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy
- PMID: 25117066
- PMCID: PMC4153454
- DOI: 10.1634/theoncologist.2014-0132
A community-based multicenter trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy
Abstract
Background: Pharmacokinetically guided (PK-guided) versus body surface area-based 5-fluorouracil (5-FU) dosing results in higher response rates and better tolerability. A paucity of data exists on PK-guided 5-FU dosing in the community setting.
Patients and methods: Seventy colorectal cancer patients, from one academic and five community cancer centers, received the mFOLFOX6 regimen (5-FU 2,400 mg/m(2) over 46 hours every 2 weeks) with or without bevacizumab at cycle 1. The 5-FU continuous-infusion dose was adjusted for cycles 2-4 using a PK-guided algorithm to achieve a literature-based target area under the concentration-time curve (AUC). The primary objective was to demonstrate that PK-guided 5-FU dosing improves the ability to achieve a target AUC within four cycles of therapy. The secondary objective was to demonstrate reduced incidence of 5-FU-related toxicities.
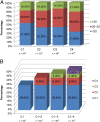
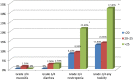
Results: At cycles 1 and 4, 27.7% and 46.8% of patients achieved the target AUC (20-25 mg × hour/L), respectively (odds ratio [OR]: 2.20; p = .046). Significantly more patients were within range at cycle 4 compared with a literature rate of 20% (p < .0001). Patients had significantly higher odds of not being underdosed at cycle 4 versus cycle 1 (OR: 2.29; p = .037). The odds of a patient being within range increased by 30% at each subsequent cycle (OR: 1.30; p = .03). Less grade 3/4 mucositis and diarrhea were observed compared with historical data (1.9% vs 16% and 5.6% vs 12%, respectively); however, rates of grade 3/4 neutropenia were similar (33% vs 25%-50%).
Conclusion: PK-guided 5-FU dosing resulted in significantly fewer underdosed patients and less gastrointestinal toxicity and allows for the application of personalized colorectal cancer therapy in the community setting.
Trial registration: ClinicalTrials.gov NCT01164215.
Keywords: Colorectal; Community; Dosing; Fluorouracil; Personalized; Pharmacokinetic.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Similar articles
-
Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study.Clin Colorectal Cancer. 2012 Dec;11(4):263-7. doi: 10.1016/j.clcc.2012.05.004. Epub 2012 Jun 9. Clin Colorectal Cancer. 2012. PMID: 22683364 Clinical Trial.
-
Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer.J Clin Oncol. 2008 May 1;26(13):2099-105. doi: 10.1200/JCO.2007.13.3934. J Clin Oncol. 2008. PMID: 18445839 Clinical Trial.
-
Prospective, Multicenter Study of 5-Fluorouracil Therapeutic Drug Monitoring in Metastatic Colorectal Cancer Treated in Routine Clinical Practice.Clin Colorectal Cancer. 2016 Dec;15(4):381-388. doi: 10.1016/j.clcc.2016.04.001. Epub 2016 May 7. Clin Colorectal Cancer. 2016. PMID: 27256667 Clinical Trial.
-
Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes.J Natl Cancer Inst. 2009 Nov 18;101(22):1543-52. doi: 10.1093/jnci/djp328. Epub 2009 Oct 19. J Natl Cancer Inst. 2009. PMID: 19841331 Review.
-
How can we best monitor 5-FU administration to maximize benefit to risk ratio?Expert Opin Drug Metab Toxicol. 2018 Dec;14(12):1303-1313. doi: 10.1080/17425255.2018.1550484. Epub 2018 Nov 23. Expert Opin Drug Metab Toxicol. 2018. PMID: 30451549 Review.
Cited by
-
Pharmacogenetics of Drugs Used in the Treatment of Cancers.Genes (Basel). 2022 Feb 7;13(2):311. doi: 10.3390/genes13020311. Genes (Basel). 2022. PMID: 35205356 Free PMC article. Review.
-
Is monitoring of plasma 5-fluorouracil levels in metastatic / advanced colorectal cancer clinically effective? A systematic review.BMC Cancer. 2016 Jul 25;16:523. doi: 10.1186/s12885-016-2581-x. BMC Cancer. 2016. PMID: 27456697 Free PMC article.
-
Evaluation of the 5-fluorouracil plasma level in patients with colorectal cancer undergoing continuous infusion chemotherapy.Mol Clin Oncol. 2019 Sep;11(3):289-295. doi: 10.3892/mco.2019.1893. Epub 2019 Jul 3. Mol Clin Oncol. 2019. PMID: 31396387 Free PMC article.
-
Therapeutic Drug Monitoring in Oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology Recommendations for 5-Fluorouracil Therapy.Clin Pharmacol Ther. 2019 Mar;105(3):598-613. doi: 10.1002/cpt.1124. Epub 2018 Sep 11. Clin Pharmacol Ther. 2019. PMID: 29923599 Free PMC article. Review.
-
Emerging roles for clinical pharmacometrics in cancer precision medicine.Curr Pharmacol Rep. 2018 Jun;4(3):276-283. doi: 10.1007/s40495-018-0139-0. Epub 2018 Apr 20. Curr Pharmacol Rep. 2018. PMID: 30345221 Free PMC article.
References
-
- Poon MA, O’Connell MJ, Wieand HS, et al. Biochemical modulation of fluorouracil with leucovorin: Confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol. 1991;9:1967–1972. - PubMed
-
- Pinedo HM, Peters GF. Fluorouracil: Biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. - PubMed
-
- de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French intergroup study. J Clin Oncol. 1997;15:808–815. - PubMed
-
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. - PubMed
-
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

