Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection
- PMID: 25117537
- PMCID: PMC4130517
- DOI: 10.1371/journal.pone.0104113
Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection
Abstract
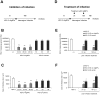
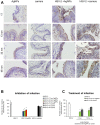
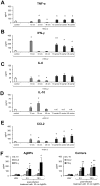
The interaction between silver nanoparticles and herpesviruses is attracting great interest due to their antiviral activity and possibility to use as microbicides for oral and anogenital herpes. In this work, we demonstrate that tannic acid modified silver nanoparticles sized 13 nm, 33 nm and 46 nm are capable of reducing HSV-2 infectivity both in vitro and in vivo. The antiviral activity of tannic acid modified silver nanoparticles was size-related, required direct interaction and blocked virus attachment, penetration and further spread. All tested tannic acid modified silver nanoparticles reduced both infection and inflammatory reaction in the mouse model of HSV-2 infection when used at infection or for a post-infection treatment. Smaller-sized nanoparticles induced production of cytokines and chemokines important for anti-viral response. The corresponding control buffers with tannic acid showed inferior antiviral effects in vitro and were ineffective in blocking in vivo infection. Our results show that tannic acid modified silver nanoparticles are good candidates for microbicides used in treatment of herpesvirus infections.
Conflict of interest statement
Figures
Similar articles
-
Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis.Viruses. 2018 Sep 26;10(10):524. doi: 10.3390/v10100524. Viruses. 2018. PMID: 30261662 Free PMC article.
-
Adjuvanticity of Tannic Acid-Modified Nanoparticles Improves Effectiveness of the Antiviral Response.Int J Nanomedicine. 2025 Apr 1;20:3977-3997. doi: 10.2147/IJN.S512509. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40191045 Free PMC article.
-
Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies.Int J Mol Sci. 2018 Jan 28;19(2):387. doi: 10.3390/ijms19020387. Int J Mol Sci. 2018. PMID: 29382085 Free PMC article.
-
Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2.Viruses. 2021 Jun 25;13(7):1228. doi: 10.3390/v13071228. Viruses. 2021. PMID: 34202050 Free PMC article. Review.
-
An overview application of silver nanoparticles in inhibition of herpes simplex virus.Artif Cells Nanomed Biotechnol. 2018 Mar;46(2):263-267. doi: 10.1080/21691401.2017.1307208. Epub 2017 Apr 12. Artif Cells Nanomed Biotechnol. 2018. PMID: 28403676 Review.
Cited by
-
Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications.Nanomaterials (Basel). 2024 Oct 10;14(20):1618. doi: 10.3390/nano14201618. Nanomaterials (Basel). 2024. PMID: 39452955 Free PMC article. Review.
-
Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19.Sustain Cities Soc. 2021 Sep;72:103046. doi: 10.1016/j.scs.2021.103046. Epub 2021 May 25. Sustain Cities Soc. 2021. PMID: 34055576 Free PMC article. Review.
-
Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis.Viruses. 2018 Sep 26;10(10):524. doi: 10.3390/v10100524. Viruses. 2018. PMID: 30261662 Free PMC article.
-
Theranostics Aspects of Various Nanoparticles in Veterinary Medicine.Int J Mol Sci. 2018 Oct 24;19(11):3299. doi: 10.3390/ijms19113299. Int J Mol Sci. 2018. PMID: 30352960 Free PMC article. Review.
-
Facile synthesis of SnO2 shell followed by microwave treatment for high environmental stability of Ag nanoparticles.RSC Adv. 2020 Oct 23;10(63):38424-38436. doi: 10.1039/d0ra06159j. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517546 Free PMC article.
References
-
- Garner JA (2003) Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv Drug Deliv Rev 55: 1497–1513. - PubMed
-
- Gupta R, Warren T, Wald A (2007) Genital herpes. Lancet 370: 2127–2137. - PubMed
-
- Halioua B, Malkin JE (1999) Epidemiology of genital herpes – recent advances. Eur J Dermatol 9: 177–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

