Phosphoproteomic profiling of human myocardial tissues distinguishes ischemic from non-ischemic end stage heart failure
- PMID: 25117565
- PMCID: PMC4130503
- DOI: 10.1371/journal.pone.0104157
Phosphoproteomic profiling of human myocardial tissues distinguishes ischemic from non-ischemic end stage heart failure
Abstract
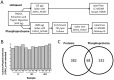
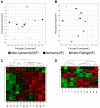
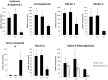
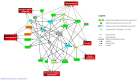
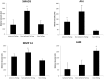
The molecular differences between ischemic (IF) and non-ischemic (NIF) heart failure are poorly defined. A better understanding of the molecular differences between these two heart failure etiologies may lead to the development of more effective heart failure therapeutics. In this study extensive proteomic and phosphoproteomic profiles of myocardial tissue from patients diagnosed with IF or NIF were assembled and compared. Proteins extracted from left ventricular sections were proteolyzed and phosphopeptides were enriched using titanium dioxide resin. Gel- and label-free nanoscale capillary liquid chromatography coupled to high resolution accuracy mass tandem mass spectrometry allowed for the quantification of 4,436 peptides (corresponding to 450 proteins) and 823 phosphopeptides (corresponding to 400 proteins) from the unenriched and phospho-enriched fractions, respectively. Protein abundance did not distinguish NIF from IF. In contrast, 37 peptides (corresponding to 26 proteins) exhibited a ≥ 2-fold alteration in phosphorylation state (p<0.05) when comparing IF and NIF. The degree of protein phosphorylation at these 37 sites was specifically dependent upon the heart failure etiology examined. Proteins exhibiting phosphorylation alterations were grouped into functional categories: transcriptional activation/RNA processing; cytoskeleton structure/function; molecular chaperones; cell adhesion/signaling; apoptosis; and energetic/metabolism. Phosphoproteomic analysis demonstrated profound post-translational differences in proteins that are involved in multiple cellular processes between different heart failure phenotypes. Understanding the roles these phosphorylation alterations play in the development of NIF and IF has the potential to generate etiology-specific heart failure therapeutics, which could be more effective than current therapeutics in addressing the growing concern of heart failure.
Conflict of interest statement
Figures



References
-
- Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, et al. (2002) Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106: 3068–3072. - PubMed
-
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, et al. (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397–1402. - PubMed
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2012) Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125: 188–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

