Integrin αvβ3 drives slug activation and stemness in the pregnant and neoplastic mammary gland
- PMID: 25117682
- PMCID: PMC4147869
- DOI: 10.1016/j.devcel.2014.06.005
Integrin αvβ3 drives slug activation and stemness in the pregnant and neoplastic mammary gland
Abstract
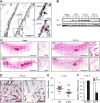
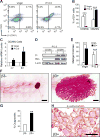
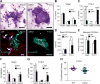
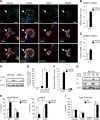
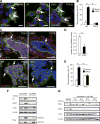
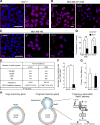
Although integrin αvβ3 is linked to cancer progression, its role in epithelial development is unclear. Here, we show that αvβ3 plays a critical role in adult mammary stem cells (MaSCs) during pregnancy. Whereas αvβ3 is a luminal progenitor marker in the virgin gland, we noted increased αvβ3 expression in MaSCs at midpregnancy. Accordingly, mice lacking αvβ3 or expressing a signaling-deficient receptor showed defective mammary gland morphogenesis during pregnancy. This was associated with decreased MaSC expansion, clonogenicity, and expression of Slug, a master regulator of MaSCs. Surprisingly, αvβ3-deficient mice displayed normal development of the virgin gland with no effect on luminal progenitors. Transforming growth factor β2 (TGF-β2) induced αvβ3 expression, enhancing Slug nuclear accumulation and MaSC clonogenicity. In human breast cancer cells, αvβ3 was necessary and sufficient for Slug activation, tumorsphere formation, and tumor initiation. Thus, pregnancy-associated MaSCs require a TGF-β2/αvβ3/Slug pathway, which may contribute to breast cancer progression and stemness.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Comment in
-
A role for β3-integrins in linking breast development and cancer.Dev Cell. 2014 Aug 11;30(3):251-2. doi: 10.1016/j.devcel.2014.07.009. Dev Cell. 2014. PMID: 25117679
References
-
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. - PubMed
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

