The lag time in initiating clinical testing of new drugs in combination with radiation therapy, a significant barrier to progress?
- PMID: 25117813
- PMCID: PMC4183859
- DOI: 10.1038/bjc.2014.448
The lag time in initiating clinical testing of new drugs in combination with radiation therapy, a significant barrier to progress?
Abstract
Background: The clinical development of new drugs with radiation appears to be limited. We hypothesised that phase I clinical trials with radiation therapy (RT) are initiated too late into a new drug's lifetime, impeding the ability to complete RT-drug development programmes before patent expiration.
Methods: We identified novel drug-radiation phase I combination trials performed between 1980 and 2012 within the PubMed and ClinicalTrials.gov databases. Data gathered for each drug included: date the initial phase I trial with/without RT was opened/published, date of the published positive phase III trials, and patent expiration dates. Lag time was defined as the interval between opening of the phase I trial without RT and the opening of the phase I with RT. Linear regression was used to model how the lag time has changed over time.
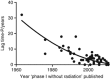
Results: The median lag time was 6 years. The initial phase I trial with RT was typically published 2 years after the first published positive phase III trial and 11 years before patent expiration. Using a best-fit linear model, lag time decreased from 10 years for phase I trials published in 1990 to 5 years in 2005 (slope significantly non-zero, P<0.001).
Conclusions: Clinical drug development with RT commences late in the life cycle of anti-cancer agents. Taking into account the additional time required for late-phase clinical trials, the delay in initiating clinical testing of drug-RT combinations discourages drug companies from further pursuing RT-based development. Encouragingly, lag time appears to be decreasing. Further reduction in lag time may accelerate RT-based drug development, potentially improving patient outcomes.
Figures

Similar articles
-
The clinical development of molecularly targeted agents in combination with radiation therapy: a pharmaceutical perspective.Int J Radiat Oncol Biol Phys. 2012 Nov 15;84(4):e447-54. doi: 10.1016/j.ijrobp.2012.05.019. Epub 2012 Jul 20. Int J Radiat Oncol Biol Phys. 2012. PMID: 22819210 Review.
-
Radiotherapy and chemotherapy in locally advanced non-small cell lung cancer: preclinical and early clinical data.Hematol Oncol Clin North Am. 2004 Feb;18(1):41-53. doi: 10.1016/s0889-8588(03)00138-2. Hematol Oncol Clin North Am. 2004. PMID: 15005280 Review.
-
INGN 201: Ad-p53, Ad5CMV-p53, adenoviral p53, p53 gene therapy--introgen, RPR/INGN 201.Drugs R D. 2007;8(3):176-87. doi: 10.2165/00126839-200708030-00005. Drugs R D. 2007. PMID: 17472413 Review.
-
Efaproxiral: GSJ 61, JP 4, KDD 86, RS 4, RSR 13.Drugs R D. 2005;6(3):178-85. doi: 10.2165/00126839-200506030-00007. Drugs R D. 2005. PMID: 15869322 Review.
-
INGN 201: Ad-p53, Ad5CMV-p53, Adenoviral p53, INGN 101, p53 gene therapy--Introgen, RPR/INGN 201.BioDrugs. 2003;17(3):216-22. doi: 10.2165/00063030-200317030-00010. BioDrugs. 2003. PMID: 12749760 Review.
Cited by
-
Repurposing Drugs for Cancer Radiotherapy: Early Successes and Emerging Opportunities.Cancer J. 2019 Mar/Apr;25(2):106-115. doi: 10.1097/PPO.0000000000000369. Cancer J. 2019. PMID: 30896532 Free PMC article. Review.
-
Challenges and opportunities for early phase clinical trials of novel drug-radiotherapy combinations: recommendations from NRG Oncology, the American Society for Radiation Oncology (ASTRO), the American College of Radiology (ACR), the Sarah Cannon Research Institute, and the American College of Radiation Oncology (ACRO).Lancet Oncol. 2024 Oct;25(10):e489-e500. doi: 10.1016/S1470-2045(24)00264-X. Lancet Oncol. 2024. PMID: 39362260 Free PMC article. Review.
-
Mechanistic Insights into Molecular Targeting and Combined Modality Therapy for Aggressive, Localized Prostate Cancer.Front Oncol. 2016 Feb 16;6:24. doi: 10.3389/fonc.2016.00024. eCollection 2016. Front Oncol. 2016. PMID: 26909338 Free PMC article. Review.
-
Clinical Development of Novel Drug-Radiotherapy Combinations.Clin Cancer Res. 2019 Mar 1;25(5):1455-1461. doi: 10.1158/1078-0432.CCR-18-2466. Epub 2018 Nov 29. Clin Cancer Res. 2019. PMID: 30498095 Free PMC article. Review.
-
Patient Derived Models to Study Head and Neck Cancer Radiation Response.Cancers (Basel). 2020 Feb 12;12(2):419. doi: 10.3390/cancers12020419. Cancers (Basel). 2020. PMID: 32059418 Free PMC article. Review.
References
-
- Ataman OU, Sambrook SJ, Wilks C, Lloyd A, Taylor AE, Wedge SR. The clinical development of molecularly targeted agents in combination with radiation therapy: a pharmaceutical perspective. Int J Radiat Oncol Biol Phys. 2012;84 (4:e447–e454. - PubMed
-
- Barton JH, Emanuel EJ. The patents-based pharmaceutical development process: rationale, problems, and potential reforms. JAMA. 2005;294 (16:2075–2082. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354 (6:567–578. - PubMed
-
- Dickersin K, Chan S, Chalmers TC, Sacks HS, Smith HJr. Publication bias and clinical trials. Control Clin Trials. 1987;8 (4:343–353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

