Genetic variant predicts bevacizumab-induced hypertension in ECOG-5103 and ECOG-2100
- PMID: 25117820
- PMCID: PMC4453857
- DOI: 10.1038/bjc.2014.430
Genetic variant predicts bevacizumab-induced hypertension in ECOG-5103 and ECOG-2100
Abstract
Background: Bevacizumab has broad anti-tumour activity, but substantial risk of hypertension. No reliable markers are available for predicting bevacizumab-induced hypertension.
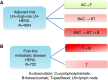
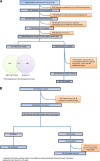
Methods: A genome-wide association study (GWAS) was performed in the phase III bevacizumab-based adjuvant breast cancer trial, ECOG-5103, to evaluate for an association between genotypes and hypertension. GWAS was conducted in those who had experienced systolic blood pressure (SBP) >160 mm Hg during therapy using binary analysis and a cumulative dose model for the total exposure of bevacizumab. Common toxicity criteria (CTC) grade 3-5 hypertension was also assessed. Candidate SNP validation was performed in the randomised phase III trial, ECOG-2100.
Results: When using the phenotype of SBP>160 mm Hg, the most significant association in SV2C (rs6453204) approached and met genome-wide significance in the binary model (P=6.0 × 10(-8); OR=3.3) and in the cumulative dose model (P=4.7 × 10(-8); HR=2.2), respectively. Similar associations with rs6453204 were seen for CTC grade 3-5 hypertension but did not meet genome-wide significance. Validation study from ECOG-2100 demonstrated a statistically significant association between this SNP and grade 3/4 hypertension using the binary model (P-value=0.037; OR=2.4).
Conclusions: A genetic variant in SV2C predicted clinically relevant bevacizumab-induced hypertension in two independent, randomised phase III trials.
Figures


References
-
- Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res. 2012;18 (18:5099–5109. - PMC - PubMed
-
- Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, Rugo HS. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29 (32:4286–4293. - PubMed
-
- Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14 (10:933–942. - PubMed
-
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2 (5:401–404. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA14958/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA049883/CA/NCI NIH HHS/United States
- U10 CA180795/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA155311-01A1/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- U10 CA014958/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- CA12027/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- R01 CA155311/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- CA39229/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
- CA49883/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

