Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy
- PMID: 25117977
- PMCID: PMC4198934
- DOI: 10.1016/j.neo.2014.06.003
Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy
Abstract
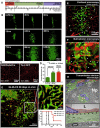
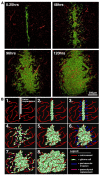
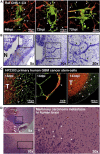
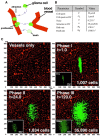
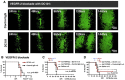
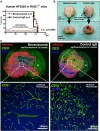
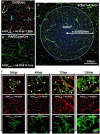
As glioma cells infiltrate the brain they become associated with various microanatomic brain structures such as blood vessels, white matter tracts, and brain parenchyma. How these distinct invasion patterns coordinate tumor growth and influence clinical outcomes remain poorly understood. We have investigated how perivascular growth affects glioma growth patterning and response to antiangiogenic therapy within the highly vascularized brain. Orthotopically implanted rodent and human glioma cells are shown to commonly invade and proliferate within brain perivascular space. This form of brain tumor growth and invasion is also shown to characterize de novo generated endogenous mouse brain tumors, biopsies of primary human glioblastoma (GBM), and peripheral cancer metastasis to the human brain. Perivascularly invading brain tumors become vascularized by normal brain microvessels as individual glioma cells use perivascular space as a conduit for tumor invasion. Agent-based computational modeling recapitulated biological perivascular glioma growth without the need for neoangiogenesis. We tested the requirement for neoangiogenesis in perivascular glioma by treating animals with angiogenesis inhibitors bevacizumab and DC101. These inhibitors induced the expected vessel normalization, yet failed to reduce tumor growth or improve survival of mice bearing orthotopic or endogenous gliomas while exacerbating brain tumor invasion. Our results provide compelling experimental evidence in support of the recently described failure of clinically used antiangiogenics to extend the overall survival of human GBM patients.
Copyright © 2014 Neoplasia Press, Inc. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63:1–35.
-
- Scherer HJ. Structural development in gliomas. Am J Cancer. 1938;34:333–351.
-
- Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57:1306–1315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01-NS082311/NS/NINDS NIH HHS/United States
- U01 NS052465/NS/NINDS NIH HHS/United States
- R01 NS061107/NS/NINDS NIH HHS/United States
- 1U01-NS052465/NS/NINDS NIH HHS/United States
- T32 CA009676/CA/NCI NIH HHS/United States
- R01 NS057711/NS/NINDS NIH HHS/United States
- R01 NS096756/NS/NINDS NIH HHS/United States
- T32 HL007622/HL/NHLBI NIH HHS/United States
- R01 NS054193/NS/NINDS NIH HHS/United States
- 1R01-NS 054193/NS/NINDS NIH HHS/United States
- R01 NS082311/NS/NINDS NIH HHS/United States
- R01 NS074387/NS/NINDS NIH HHS/United States
- 1R01-NS 057711/NS/NINDS NIH HHS/United States
- 1R01-NS 061107/NS/NINDS NIH HHS/United States
- 1R01-NS074387/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

