Follicle stimulating hormone receptor in mesenchymal stem cells integrates effects of glycoprotein reproductive hormones
- PMID: 25118101
- PMCID: PMC4289445
- DOI: 10.1111/nyas.12502
Follicle stimulating hormone receptor in mesenchymal stem cells integrates effects of glycoprotein reproductive hormones
Abstract
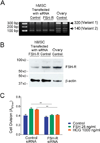
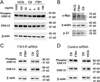
Previously we reported that follicle stimulating hormone (FSH) affects bone degradation in human cells and in follicle stimulating hormone receptor (FSH-R) null mice. Here we describe a FSH-R knockout bone-formation phenotype. We used mesenchymal stem cells (MSCs), osteoblast precursors that express FSH-R, to determine whether FSH regulates bone formation. FSH stimulates MSC cell adhesion 1-3 h and proliferation at 24 h after addition. On the basis of phylogenetic and clinical precedents, we also examined effects of pregnant levels of human chorionic gonadotropin (hCG) on MSCs. We found effects similar to those of FSH, and RNAi knockdown of FSH-R abrogated both FSH and hCG effects on MSCs. In contrast to effects on MSCs, neither FSH nor hCG had significant effects on osteoblast maturation. Also in MSCs, short-term treatment by FSH and hCG altered signaling pathways for proliferation, including Erk1/2 phosphorylation. Our results show augmentation of MSC proliferation by either FSH at menopausal levels or hCG at normal pregnant levels. We conclude that FSH-R participates in regulation of MSC precursor pools in response to either FSH or hCG, integrating the effects of these two glycoprotein hormones.
Keywords: Erk1/2; FSH; hCG; osteoblast; osteoporosis.
© 2014 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Restoration of human chorionic gonadotropin response in human myometrial smooth muscle cells by treatment with follicle-stimulating hormone (FSH): evidence for the presence of FSH receptors in human myometrium.Eur J Endocrinol. 1996 Feb;134(2):225-31. doi: 10.1530/eje.0.1340225. Eur J Endocrinol. 1996. PMID: 8630524
-
Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro.Mol Cell Endocrinol. 2016 Feb 15;422:103-114. doi: 10.1016/j.mce.2015.12.008. Epub 2015 Dec 12. Mol Cell Endocrinol. 2016. PMID: 26690776
-
Lys91 and His90 of the alpha-subunit are crucial for receptor binding and hormone action of follicle-stimulating hormone (FSH) and play hormone-specific roles in FSH and human chorionic gonadotropin.Endocrinology. 1995 Jul;136(7):2948-53. doi: 10.1210/endo.136.7.7540545. Endocrinology. 1995. PMID: 7540545
-
Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy?Int J Mol Sci. 2023 May 19;24(10):9020. doi: 10.3390/ijms24109020. Int J Mol Sci. 2023. PMID: 37240364 Free PMC article. Review.
-
Glycoprotein G-protein Coupled Receptors in Disease: Luteinizing Hormone Receptors and Follicle Stimulating Hormone Receptors.Diseases. 2020 Sep 15;8(3):35. doi: 10.3390/diseases8030035. Diseases. 2020. PMID: 32942611 Free PMC article. Review.
Cited by
-
The ecto-nucleotide pyrophosphatase/phosphodiesterase 2 promotes early osteoblast differentiation and mineralization in stromal stem cells.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C843-C849. doi: 10.1152/ajpcell.00692.2023. Epub 2024 Jan 15. Am J Physiol Cell Physiol. 2024. PMID: 38223929 Free PMC article.
-
DEAD-Box Helicase 4 (Ddx4)+ Stem Cells Sustain Tumor Progression in Non-Serous Ovarian Cancers.Int J Mol Sci. 2020 Aug 24;21(17):6096. doi: 10.3390/ijms21176096. Int J Mol Sci. 2020. PMID: 32847044 Free PMC article.
-
Live imaging of follicle stimulating hormone receptors in gonads and bones using near infrared II fluorophore.Chem Sci. 2017 May 1;8(5):3703-3711. doi: 10.1039/c6sc04897h. Epub 2017 Mar 6. Chem Sci. 2017. PMID: 28626555 Free PMC article.
-
Follicle-Stimulating Hormone Increases the Risk of Postmenopausal Osteoporosis by Stimulating Osteoclast Differentiation.PLoS One. 2015 Aug 4;10(8):e0134986. doi: 10.1371/journal.pone.0134986. eCollection 2015. PLoS One. 2015. PMID: 26241313 Free PMC article.
-
FSH, Bone Mass, Body Fat, and Biological Aging.Endocrinology. 2018 Oct 1;159(10):3503-3514. doi: 10.1210/en.2018-00601. Endocrinology. 2018. PMID: 30085049 Free PMC article.
References
-
- Abe E, Marians RC, Yu W, et al. TSH Is a Negative Regulator of Skeletal Remodeling. Cell. 2003;115:151–162. - PubMed
-
- Sun L, Peng Y, Sharrow AC AC, et al. FSH Directly Regulates Bone Mass. Cell. 2006;125:247–260. - PubMed
-
- Vibede N, Hauser M, Williamson CJ, Grimmelikhuijzen F. Genomic Organization of a Receptor from Sea Anemones, Structurally and Evolutionarily Related to Glycoprotein Hormone Receptors from Mammals. Biochem. Biophys. Res. Commun. 1998;252:497–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

