Effect of guided self-determination youth intervention integrated into outpatient visits versus treatment as usual on glycemic control and life skills: a randomized clinical trial in adolescents with type 1 diabetes
- PMID: 25118146
- PMCID: PMC4247629
- DOI: 10.1186/1745-6215-15-321
Effect of guided self-determination youth intervention integrated into outpatient visits versus treatment as usual on glycemic control and life skills: a randomized clinical trial in adolescents with type 1 diabetes
Abstract
Background: Providing care for adolescents with type 1 diabetes is complex, demanding, and often unsuccessful. Guided self-determination (GSD) is a life skills approach that has been proven effective in caring for adults with type 1 diabetes. To improve care, GSD was revised for adolescents, their parents, and interdisciplinary healthcare providers (HCP) to create GSD-Youth (GSD-Y). We evaluated the impact of GSD-Y after it was integrated into pediatric outpatient visits versus treatment-as-usual, focusing on glycemic control and the development of life skills in adolescents with type 1 diabetes.
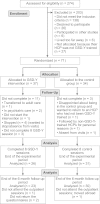
Methods: Seventy-one adolescents (mean age: 15 years, mean duration of diabetes: 5.7 years, mean HbA1c: 77 mmol/mol (9.1%), upon entering the study) from two pediatric departments were randomized into a GSD-Y group (n = 37, GSD-Y was provided during individual outpatient sessions) versus a treatment-as-usual group (n = 34). The primary outcome was the HbA1c measurement. The secondary outcomes were life skills development (assessed by self-reported psychometric scales), self-monitored blood glucose levels, and hypo- and hyperglycemic episodes. The analysis followed an intention-to-treat basis.
Results: Fifty-seven adolescents (80%) completed the trial, and 53 (75%) completed a six-month post-treatment follow-up. No significant effect of GSD-Y on the HbA1c could be detected in a mixed-model analysis after adjusting for the baseline HbA1c levels and the identity of the HCP (P = 0.85). GSD-Y significantly reduced the amotivation for diabetes self-management after adjusting for the baseline value (P = 0.001). Compared with the control group, the trial completion was prolonged in the GSD-Y group (P <0.001), requiring more visits (P = 0.05) with a higher rate of non-attendance (P = 0.01). GSD-Y parents participated in fewer of the adolescents' visits (P = 0.05) compared with control parents.
Conclusions: Compared with treatment-as-usual, GSD-Y did not improve HbA1c levels, but it did decrease adolescents' amotivation for diabetes self-management.
Trial registration: ISRCTN 54243636, registered on 10 January 2010. Life skills for adolescents with type 1 diabetes and their parents.
Figures
References
-
- Cameron FJ, Skinner TC, de Beaufort CE, Hoey H, Swift PG, Aanstoot H, Aman J, Martul P, Chiarelli F, Daneman D, Danne T, Dorchy H, Kaprio EA, Kaufman F, Kocova M, Mortensen HB, Njølstad PR, Phillip M, Robertson KJ, Schoenle EJ, Urakami T, Vanelli M, Ackermann RW, Skovlund SE, Hvidoere Study Group on Childhood Diabetes Are family factors universally related to metabolic outcomes in adolescents with Type 1 diabetes? Diabet Med. 2008;25:463–468. doi: 10.1111/j.1464-5491.2008.02399.x. - DOI - PubMed
-
- White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV, Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT) J Pediatr. 2001;139:804–812. doi: 10.1067/mpd.2001.118887. - DOI - PubMed
-
- Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ, International Society for Pediatric and Adolescent Diabetes (ISBAD) Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2007;8:408–418. doi: 10.1111/j.1399-5448.2007.00352.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous