Immature truncated O-glycophenotype of cancer directly induces oncogenic features
- PMID: 25118277
- PMCID: PMC4191756
- DOI: 10.1073/pnas.1406619111
Immature truncated O-glycophenotype of cancer directly induces oncogenic features
Abstract
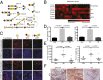
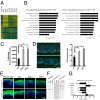
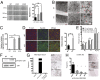
Aberrant expression of immature truncated O-glycans is a characteristic feature observed on virtually all epithelial cancer cells, and a very high frequency is observed in early epithelial premalignant lesions that precede the development of adenocarcinomas. Expression of the truncated O-glycan structures Tn and sialyl-Tn is strongly associated with poor prognosis and overall low survival. The genetic and biosynthetic mechanisms leading to accumulation of truncated O-glycans are not fully understood and include mutation or dysregulation of glycosyltransferases involved in elongation of O-glycans, as well as relocation of glycosyltransferases controlling initiation of O-glycosylation from Golgi to endoplasmic reticulum. Truncated O-glycans have been proposed to play functional roles for cancer-cell invasiveness, but our understanding of the biological functions of aberrant glycosylation in cancer is still highly limited. Here, we used exome sequencing of most glycosyltransferases in a large series of primary and metastatic pancreatic cancers to rule out somatic mutations as a cause of expression of truncated O-glycans. Instead, we found hypermethylation of core 1 β3-Gal-T-specific molecular chaperone, a key chaperone for O-glycan elongation, as the most prevalent cause. We next used gene editing to produce isogenic cell systems with and without homogenous truncated O-glycans that enabled, to our knowledge, the first polyomic and side-by-side evaluation of the cancer O-glycophenotype in an organotypic tissue model and in xenografts. The results strongly suggest that truncation of O-glycans directly induces oncogenic features of cell growth and invasion. The study provides support for targeting cancer-specific truncated O-glycans with immunotherapeutic measures.
Keywords: epigenetics; glycans; keratinocyte; pancreas; skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Not so sweet malignant transformation.Proc Natl Acad Sci U S A. 2014 Sep 30;111(39):14009-10. doi: 10.1073/pnas.1415576111. Epub 2014 Sep 16. Proc Natl Acad Sci U S A. 2014. PMID: 25228771 Free PMC article. No abstract available.
References
-
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. - PubMed
-
- Springer GFT. T and Tn, general carcinoma autoantigens. Science. 1984;224(4654):1198–1206. - PubMed
-
- Itzkowitz S, et al. Expression of Tn, sialosyl Tn, and T antigens in human pancreas. Gastroenterology. 1991;100(6):1691–1700. - PubMed
-
- Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780(3):546–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

