Glutathione S-transferase M1 modulates allergen-induced NF-κB activation in asthmatic airway epithelium
- PMID: 25118837
- PMCID: PMC4317365
- DOI: 10.1111/all.12506
Glutathione S-transferase M1 modulates allergen-induced NF-κB activation in asthmatic airway epithelium
Abstract
Background: Glutathione S-transferase M1 (GSTM1) is a phase II enzyme and regulator of inflammatory signaling in airway epithelial cells. We have found upregulation of neutrophilic airway inflammation in atopic asthmatics expressing GSTM1 gene (GSTM1+) compared to GSTM1null asthmatics. We hypothesized that GSTM1 modulates NF-κB activation in bronchial epithelium in atopic asthmatics. We determined regulation of allergen-induced NF-κB activation in bronchial epithelium by GSTM1 in human atopic asthmatics in vivo.
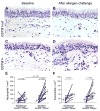
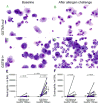
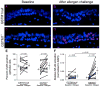
Methods: Endobronchial biopsies and bronchoalveolar lavage fluid samples were collected from 13 GSTM1+ and 12 GSTM1null human atopic asthmatics at baseline and 24 h after segmental allergen challenge. A quantitative analysis of NF-κB activation in airway epithelium was accomplished using a polyclonal antibody against the phosphorylated p65 component of NF-κB. Elastase-positive neutrophils in the bronchial wall were quantified.
Results: Postallergen neutrophilia in airway subepithelium and epithelial lining fluid was greater in GSTM1+ compared to GSTM1null asthmatics. Airway eosinophilia was similar in GSTM1+ and GSTM1null asthmatics. Allergen-provoked NF-κB induction in bronchial epithelium was significantly greater in GSTM1+ compared to GSTM1null asthmatics. Activation of NF-κB activation in airway epithelial cells correlated with interleukin-8 concentrations and absolute neutrophil numbers in bronchoalveolar lavage fluid in GSTM1+ but not GSTM1null asthmatics.
Conclusions: Allergen-induced neutrophilic airway inflammation in GSTM1+ asthmatics is associated with NF-κB activation in airway epithelial cells in vivo. These novel data provide a potential mechanism of the genomic link between GSTM1 polymorphism and airway neutrophilia in atopic asthma.
Keywords: GSTM1 polymorphism; NF-κB; airway epithelium; atopic asthma; neutrophilic airway inflammation.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures

References
-
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. - PubMed
-
- McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC, et al. Common deletion polymorphisms in the human genoms. Nature Genetics. 2012;38:86–92. - PubMed
-
- Anttila S, Hirvonen A, Vainio H, Husgafvel-Pursiainen K, Hayes JD, Ketterer B. Immunohistochemical localization of glutathione S-transferases in the lung. Cancer Res. 1993;53:5643–8. - PubMed
-
- Cho S-G, Lee YH, Park H-S, Ryoo K, Kang KW, Park J, Eom S-J, et al. Glutathione S-Transferase Mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;16:12749–55. - PubMed
-
- Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J Biol Chem. 2002;277:30792–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

