RAB11-mediated trafficking in host-pathogen interactions
- PMID: 25118884
- PMCID: PMC4274738
- DOI: 10.1038/nrmicro3325
RAB11-mediated trafficking in host-pathogen interactions
Abstract
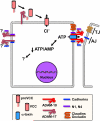
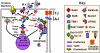
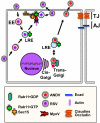
Many bacterial and viral pathogens block or subvert host cellular processes to promote successful infection. One host protein that is targeted by invading pathogens is the small GTPase RAB11, which functions in vesicular trafficking. RAB11 functions in conjunction with a protein complex known as the exocyst to mediate terminal steps in cargo transport via the recycling endosome to cell-cell junctions, phagosomes and cellular protrusions. These processes contribute to host innate immunity by promoting epithelial and endothelial barrier integrity, sensing and immobilizing pathogens and repairing pathogen-induced cellular damage. In this Review, we discuss the various mechanisms that pathogens have evolved to disrupt or subvert RAB11-dependent pathways as part of their infection strategy.
Figures



Similar articles
-
Anthrax edema toxin disrupts distinct steps in Rab11-dependent junctional transport.PLoS Pathog. 2017 Sep 25;13(9):e1006603. doi: 10.1371/journal.ppat.1006603. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28945820 Free PMC article.
-
Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst.Nature. 2010 Oct 14;467(7317):854-8. doi: 10.1038/nature09446. Nature. 2010. PMID: 20944747 Free PMC article.
-
Interaction of microbial pathogens with host exocytic pathways.Cell Microbiol. 2018 Aug;20(8):e12861. doi: 10.1111/cmi.12861. Epub 2018 Jun 20. Cell Microbiol. 2018. PMID: 29797532 Review.
-
Rab GTPases in Immunity and Inflammation.Front Cell Infect Microbiol. 2017 Sep 29;7:435. doi: 10.3389/fcimb.2017.00435. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29034219 Free PMC article. Review.
-
Listeria monocytogenes exploits host exocytosis to promote cell-to-cell spread.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3789-3796. doi: 10.1073/pnas.1916676117. Epub 2020 Feb 3. Proc Natl Acad Sci U S A. 2020. PMID: 32015134 Free PMC article.
Cited by
-
Influenza virus genome reaches the plasma membrane via a modified endoplasmic reticulum and Rab11-dependent vesicles.Nat Commun. 2017 Nov 9;8(1):1396. doi: 10.1038/s41467-017-01557-6. Nat Commun. 2017. PMID: 29123131 Free PMC article.
-
Micropatterned macrophage analysis reveals global cytoskeleton constraints induced by Bacillus anthracis edema toxin.Infect Immun. 2015 Aug;83(8):3114-25. doi: 10.1128/IAI.00479-15. Epub 2015 May 26. Infect Immun. 2015. PMID: 26015478 Free PMC article.
-
Targeting of the hydrophobic metabolome by pathogens.Traffic. 2015 May;16(5):439-60. doi: 10.1111/tra.12280. Traffic. 2015. PMID: 25754025 Free PMC article. Review.
-
Identification of Host Factors Involved in Human Cytomegalovirus Replication, Assembly, and Egress Using a Two-Step Small Interfering RNA Screen.mBio. 2018 Jun 26;9(3):e00716-18. doi: 10.1128/mBio.00716-18. mBio. 2018. PMID: 29946045 Free PMC article.
-
Evaluation of the relationship between the 14-3-3ε protein and LvRab11 in the shrimp Litopenaeus vannamei during WSSV infection.Sci Rep. 2021 Sep 28;11(1):19188. doi: 10.1038/s41598-021-97828-w. Sci Rep. 2021. PMID: 34584112 Free PMC article.
References
-
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. - PubMed
-
- Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40:1360–7. - PubMed
-
- Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

